Image copyright: Nature.
Unlocking opioid neuropeptide dynamics with genetically-encoded biosensors
Published in Nature Neuroscience.
Authors
Lin Tian, Chunyang Dong, Raajaram Gowrishankar, Yihan Jin, Xinyi He, Achla Gupta, Huikun Wang, Nilufer Atasoy, Rodolfo Flores-Garcia, Karan Mahe, Ruqiang Liang, Grace Or, Darren Lo, Qingtao Sun, Jennifer Whistler, Bo Li, Ivone Gomes, Hugo Tejeda, Deniz Atasoy, Lakshmi Devi, Michael Bruchas, Matthew Banghart
Paper presented by Dr. Renata Marchette and selected by the NIDA TDI Paper of the Month Committee
Publication Brief Description
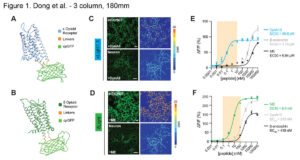
Studies in opioid neuropeptide systems have historically faced challenges due to a lack of sensitive experimental tools capable of facilitating the understanding of the complexity and diversity of endogenous opioid signaling in a circuit-specific manner. To address this, researchers have developed a class of genetically encoded opioid peptide indicators: κLight, δLight, and µLight, based on κ, δ, and µ opioid receptors, respectively. These sensors respond to both endogenous opioid neuropeptides and exogenous drugs targeting the three receptors, detecting and differentiating conformational changes in the receptors. κLight and δLight, in particular, can detect rapid opioid peptide release in a subregion-specific manner in awake and freely behaving mice. These sensors are invaluable for understanding endogenous opioid transmission in contexts such as drug addiction, withdrawal, and motivated behavior.
Unlocking opioid neuropeptide dynamics with genetically encoded biosensors Journal Article
In: Nat Neurosci, 2024, ISSN: 1546-1726.
