 Featured Paper of the Month – November 2024
Featured Paper of the Month – November 2024
Published in Neuropsychopharmacology by Flavia Barbano and Marisela Morales, et al. of the NIDA IRP Neuronal Networks Section.
Summary
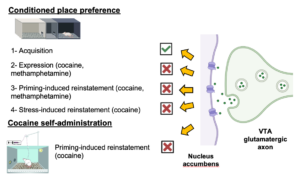
The mesocorticolimbic dopamine system is essential for psychostimulant-related behaviors like conditioned place preference (CPP), self-administration, and relapse after extinction. Centered on dopamine neurons in the ventral tegmental area (VTA) and their connections to the nucleus accumbens (NAc), this system drives psychostimulant reward responses. Our study shows that activating a parallel VTA-originating glutamatergic pathway targeting the NAc shell can suppress psychostimulant-seeking behavior. Activation of the VTA-NAc glutamatergic pathway did not affect cocaine CPP acquisition but blocked the expression, stress- and priming-induced reinstatement of cocaine and methamphetamine CPP, and cocaine-seeking in a self-administration model. Unlike the dopamine system, this VTA-NAc glutamatergic pathway suppresses psychostimulant-seeking, presenting a potential target for reducing relapse in psychostimulant users.
Publication Information
VTA glutamatergic projections to the nucleus accumbens suppress psychostimulant-seeking behavior Journal Article
In: Neuropsychopharmacology, vol. 49, no. 12, pp. 1905–1915, 2024, ISSN: 1740-634X.
