Image copyright – Nature
Customizable, wireless and implantable neural probe design and fabrication via 3D printing
Published in Nature Protocols (2022)
Authors
Kyle E Parker, Juhyun Lee, Jenny R Kim, Chinatsu Kawakami, Choong Yeon Kim, Raza Qazi, Kyung-In Jang, Jae-Woong Jeong, Jordan G McCall
Paper presented by Dr. Nicholas Beacher and selected by the NIDA TDI Paper of the Month Committee.
Publication Brief Description
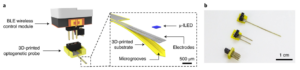
Optogenetics uses genetically encoded proteins to manipulate cellular activities via photostimulation at particular wavelengths of light. The light sources for activating optogenetic proteins can require expensive probes and equipment that prohibit large scale experiments. Furthermore, limitations of using commercial sourced probe shanks can make ‘multi-region’ optogenetics experiments in the brain challenging. Parker*, Lee*, Kim* et. al. provides a protocol including all procedures, designs, and product information for researchers to establish wireless optogenetics in their lab for low cost and high subject output. The paper details the following: 1) a custom manufacturing technique for 3D-printing large number of optogenetic probes of any length and number of ‘shanks’ in ~2 days, 2) a low-cost, custom, wireless harnesses, and 3) software for a smartphone app to control the timing and frequency of delivered light. The described technology can readily be applied for social interaction experiments where large groups of rats or mice need to be group-housed and implanted with probes in specific brain regions of interest. Wirelessly exciting or inhibiting specific neuronal subtypes in multiple regions and analyzing the associated behavioral changes using deep learning software may provide insight into the neural mechanisms involved in social interaction and clinically relevant social behaviors.
Customizable, wireless and implantable neural probe design and fabrication via 3D printing Journal Article
In: Nat Protoc, vol. 18, no. 1, pp. 3–21, 2023, ISSN: 1750-2799.
