Published in Theranostics (2023)
Authors
Alec J. Batts, Robin Ji, Rebecca L. Noel, Alina R. Kline-Schoder, Sua Bae, Nancy Kwon, and Elisa E. Konofagou
Paper presented by Dr. Brandon Harvey and selected by the NIDA TDI Paper of the Month Committee
Publication Brief Description
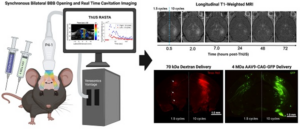
Focused ultrasound (FUS) has emerged as a versatile method to modify the brain including tumor ablation, neuromodulation and blood-brain-barrier (BBB) disruption. FUS-mediated disruption of the BBB has been used to focally target drugs and genetic material into the brain from the periphery in a minimally invasive manner. The current study from the Konofagou lab describes an advancement in FUS delivery parameters and instrumentation that allows the simultaneous opening and monitoring of BBB in two brain regions simultaneously. This preclinical study highlights new developments in FUS that expand its capabilities for safe, non-invasive gene and drug delivery to the brain for the potential treatment of neurological diseases. FUS may be a useful tool for evaluating therapeutics for substance use disorder.
In: Theranostics, vol. 13, no. 3, pp. 1180–1197, 2023, ISSN: 1838-7640.