Published in Nature (2019)
Authors
Xiaozhou Luo, Michael A Reiter, Leo d’Espaux, Jeff Wong, Charles M Denby, Anna Lechner, Yunfeng Zhang, Adrian T Grzybowski, Simon Harth, Weiyin Lin, Hyunsu Lee, Changhua Yu, John Shin, Kai Deng, Veronica T Benites, George Wang, Edward E K Baidoo, Yan Chen, Ishaan Dev, Christopher J Petzold, Jay D Keasling
Paper presented by Dr. Nicholas Beacher and selected by the NIDA TDI Paper of the Month Committee
Publication Brief Description
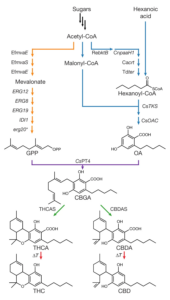
Yeast have been utilized for thousands of years to generate alcohol from simple sugars. Modern advancements in bioengineering have enabled researchers to harness similar processes and generate other substances. Luo & Reiter et. al. created publicly available strains of bioengineered yeasts (yCAN53, yCAN40, yCAN31, and others) capable of synthesizing compounds associated with cannabis from the simple sugar (galactose). Included in these compounds is an array of precursor molecules (e.g., CBGa) as well as compounds (e.g. THCa & CBDa) that convert to THC and CBD with heat (i.e., when smoked or vaporized). Feeding additional acids (e.g., hexanoic acid, heptanoic acid, 6-heptynoic acid, etc.) to the different yeast strains resulted in modified c3 side chains of THCa (yCAN40) and CBDa (yCAN31) which produced a host of novel and unexplored compounds. The paper details the methodology for creating the yeast over multiple generations and rationale for each stage. This work may aid the study of substances that only exist in small quantities in the cannabis plant (e.g., CBGa) while also identifying synthetic cannabinoids that do not exist in nature. The resulting compounds could also be explored in the context of addiction and pharmacotherapies using cell and animal models.
Complete biosynthesis of cannabinoids and their unnatural analogues in yeast Journal Article
In: Nature, vol. 567, no. 7746, pp. 123–126, 2019, ISSN: 1476-4687.