Hot Off the Press! – January 2017
Pignatelli, Marco; Umanah, George Kwabena Essien; Ribeiro, Sissi Palma; Chen, Rong; Karuppagounder, Senthilkumar Senthil; Yau, Hau-Jie; Eacker, Stephen; Dawson, Valina Lynn; Dawson, Ted Murray; Bonci, Antonello
Synaptic Plasticity onto Dopamine Neurons Shapes Fear Learning. Journal Article
In: Neuron, vol. 93, no. 2, pp. 425–440, 2017, ISSN: 1097-4199 (Electronic); 0896-6273 (Linking).
@article{Pignatelli2017,
title = {Synaptic Plasticity onto Dopamine Neurons Shapes Fear Learning.},
author = {Marco Pignatelli and George Kwabena Essien Umanah and Sissi Palma Ribeiro and Rong Chen and Senthilkumar Senthil Karuppagounder and Hau-Jie Yau and Stephen Eacker and Valina Lynn Dawson and Ted Murray Dawson and Antonello Bonci},
url = {https://www.ncbi.nlm.nih.gov/pubmed/28103482},
doi = {10.1016/j.neuron.2016.12.030},
issn = {1097-4199 (Electronic); 0896-6273 (Linking)},
year = {2017},
date = {2017-01-18},
journal = {Neuron},
volume = {93},
number = {2},
pages = {425--440},
address = {Intramural Research Program, Synaptic Plasticity Section, National Institute on Drug Abuse, Baltimore, MD 21224, USA.},
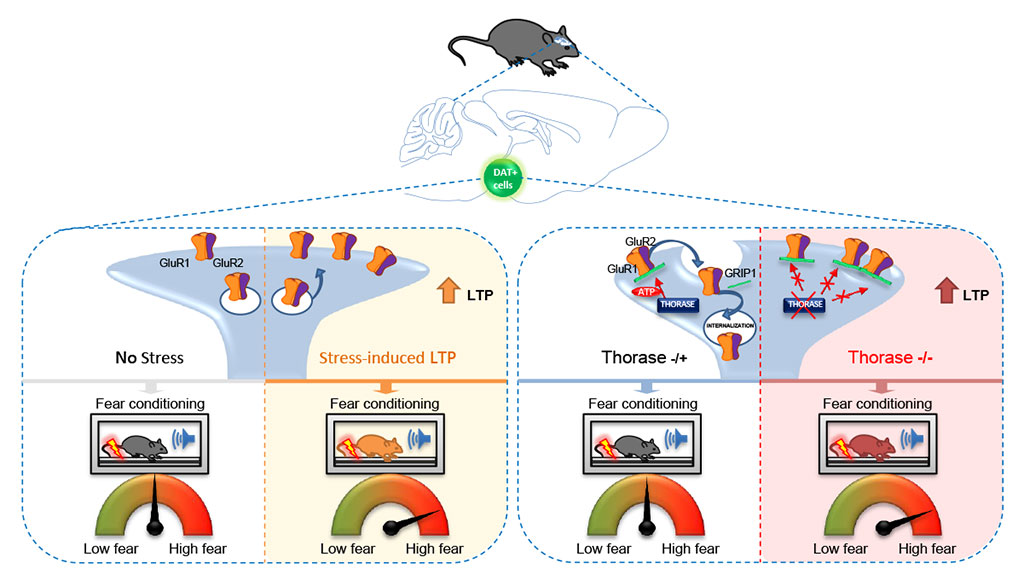
abstract = {Fear learning is a fundamental behavioral process that requires dopamine (DA) release. Experience-dependent synaptic plasticity occurs on DA neurons while an organism is engaged in aversive experiences. However, whether synaptic plasticity onto DA neurons is causally involved in aversion learning is unknown. Here, we show that a stress priming procedure enhances fear learning by engaging VTA synaptic plasticity. Moreover, we took advantage of the ability of the ATPase Thorase to regulate the internalization of AMPA receptors (AMPARs) in order to selectively manipulate glutamatergic synaptic plasticity on DA neurons. Genetic ablation of Thorase in DAT+ neurons produced increased AMPAR surface expression and function that lead to impaired induction of both long-term depression (LTD) and long-term potentiation (LTP). Strikingly, animals lacking Thorase in DAT+ neurons expressed greater associative learning in a fear conditioning paradigm. In conclusion, our data provide a novel, causal link between synaptic plasticity onto DA neurons and fear learning.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Fear learning is a fundamental behavioral process that requires dopamine (DA) release. Experience-dependent synaptic plasticity occurs on DA neurons while an organism is engaged in aversive experiences. However, whether synaptic plasticity onto DA neurons is causally involved in aversion learning is unknown. Here, we show that a stress priming procedure enhances fear learning by engaging VTA synaptic plasticity. Moreover, we took advantage of the ability of the ATPase Thorase to regulate the internalization of AMPA receptors (AMPARs) in order to selectively manipulate glutamatergic synaptic plasticity on DA neurons. Genetic ablation of Thorase in DAT+ neurons produced increased AMPAR surface expression and function that lead to impaired induction of both long-term depression (LTD) and long-term potentiation (LTP). Strikingly, animals lacking Thorase in DAT+ neurons expressed greater associative learning in a fear conditioning paradigm. In conclusion, our data provide a novel, causal link between synaptic plasticity onto DA neurons and fear learning.