Position
Senior Associate Scientist,
Magnetic Resonance Imaging and Spectroscopy Section
Adjunct professor,
Department of Computer Science and Electrical Engineering, University of Maryland, Baltimore County
Adjunct Associate Professor (2024),
Department of Psychiatry, School of Medicine, Dartmouth College
Contact
Biomedical Research Center251 Bayview Boulevard
Baltimore, MD 21224
Phone: 667-312- 5201
Email: luha@intra.nida.nih.gov
Education
B.S. - Biomedical Engineering, HuaZhong University of Science and Technology
Ph.D. - Biophysics (on MRI), Medical College of Wisconsin
Research Interests
Dr. Lu’s research centers on two areas: 1) developing new neuromodulation and neuroimaging tools, in particular, focal transcranial magnetic stimulation (TMS) and fMRI; 2) applying these tools to better understand the effects of abused drugs on the brain.
Our lab developed the imaging methodology (including the anesthetic regime), which allowed us among the first to report resting state functional connectivity in rat brains. With our collaborators at UMBC, we recently invented rodent specific focal TMS coils. Ongoing instrumentational development includes new TMS paradigms, TMS high power electronics and control, TMS coil design, MRI gradient coil design and pulse sequence development etc. Recent projects include:
- TMS technology development and its application to drug addiction treatment
- Systems-level neuroadaptation following prolonged drug exposure
- Biophysical basis of resting state fMRI signal using multiple modalities, including electrophysiological recording, optical imaging and fMRI in rodent models
Publications
Selected Publications
2024
Nguyen, Hieu; Li, Charlotte Qiong; Hoffman, Samantha; Deng, Zhi-De; Yang, Yihong; Lu, Hanbing
Ultra-high frequency repetitive TMS at subthreshold intensity induces suprathreshold motor response via temporal summation Journal Article
In: J Neural Eng, vol. 21, no. 4, 2024, ISSN: 1741-2552.
@article{pmid39079555,
title = {Ultra-high frequency repetitive TMS at subthreshold intensity induces suprathreshold motor response via temporal summation},
author = {Hieu Nguyen and Charlotte Qiong Li and Samantha Hoffman and Zhi-De Deng and Yihong Yang and Hanbing Lu},
doi = {10.1088/1741-2552/ad692f},
issn = {1741-2552},
year = {2024},
date = {2024-08-01},
urldate = {2024-08-01},
journal = {J Neural Eng},
volume = {21},
number = {4},
abstract = {The transcranial magnetic stimulation (TMS) coil induces an electric field that diminishes rapidly upon entering the brain. This presents a challenge in achieving focal stimulation of a deep brain structure. Neuronal elements, including axons, dendrites, and cell bodies, exhibit specific time constants. When exposed to repetitive TMS pulses at a high frequency, there is a cumulative effect on neuronal membrane potentials, resulting in temporal summation. This study aims to determine whether TMS pulse train at high-frequency and subthreshold intensity could induce a suprathreshold response.As a proof of concept, we developed a TMS machine in-house that could consistently output pulses up to 250 Hz, and performed experiments on 22 awake rats to test whether temporal summation was detectable under pulse trains at 100, 166, or 250 Hz.Results revealed that TMS pulses at 55% maximum stimulator output (MSO, peak d/d= 68.5 A/s at 100% MSO, pulse width = 48s) did not induce motor responses with either single pulses or pulse trains. Similarly, a single TMS pulse at 65% MSO failed to evoke a motor response in rats; however, a train of TMS pulses at frequencies of 166 and 250 Hz, but not at 100 Hz, successfully triggered motor responses and MEP signals, suggesting a temporal summation effect dependent on both pulse intensities and pulse train frequencies.We propose that the temporal summation effect can be leveraged to design the next-generation focal TMS system: by sequentially driving multiple coils at high-frequency and subthreshold intensity, areas with the most significant overlapping E-fields undergo maximal temporal summation effects, resulting in a suprathreshold response.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
2023
Nguyen, Hieu; Makaroff, Sergey N; Li, Charlotte Qiong; Hoffman, Samantha; Yang, Yihong; Lu, Hanbing
High inductance magnetic-core coils have enhanced efficiency in inducing suprathreshold motor response in rats Journal Article
In: Phys Med Biol, vol. 68, no. 24, 2023, ISSN: 1361-6560.
@article{pmid37949063,
title = {High inductance magnetic-core coils have enhanced efficiency in inducing suprathreshold motor response in rats},
author = {Hieu Nguyen and Sergey N Makaroff and Charlotte Qiong Li and Samantha Hoffman and Yihong Yang and Hanbing Lu},
doi = {10.1088/1361-6560/ad0bde},
issn = {1361-6560},
year = {2023},
date = {2023-12-01},
urldate = {2023-12-01},
journal = {Phys Med Biol},
volume = {68},
number = {24},
abstract = {. Transcranial magnetic stimulation (TMS) coil design involves a tradeoff among multiple parameters, including magnetic flux density (), inductance (), induced electric () field, focality, penetration depth, coil heating, etc. Magnetic materials with high permeability have been suggested to enhance coil efficiency. However, the introduction of magnetic core invariably increases coil inductance compared to its air-core counterpart, which in turn weakens thefield. Our lab previously reported a rodent-specific TMS coil with silicon steel magnetic core, achieving 2 mm focality. This study aims to better understand the tradeoffs among,andin the presence of magnetic core.. The magnetic core initially operates within the linear range, transitioning to the nonlinear range when it begins to saturate at high current levels and reverts to the linear range as coil current approaches zero; both linear and nonlinear analyses were performed. Linear analysis assumes a weak current condition when magnetic core is not saturated; a monophasic TMS circuit was employed for this purpose. Nonlinear analysis assumes a strong current condition with varying degrees of core saturation.. Results reveal that, the secondaryfield generated by the silicon steel core substantially changed the dynamics during TMS pulse. Linear and nonlinear analyses revealed that higher inductance coils produced stronger peakfields and longerfield waveforms. On a macroscopic scale, the effects of these two factors on neuronal activation could be conceptually explained through a one-time-constant linear membrane model. Four coils with different,andcharacteristics were designed and constructed. Bothfield mapping and experiments on awake rats confirmed that inductance could be much higher than previously anticipated, provided that magnetic material possesses a high saturation threshold.. Our results highlight the novel potentials of magnetic core in TMS coil designs, especially for small animals.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
2022
Meng, Qinglei; Nguyen, Hieu; Vrana, Antonia; Baldwin, Simone; Li, Charlotte Qiong; Giles, Antonia; Wang, Jun; Yang, Yihong; Lu, Hanbing
In: Brain Stimul, vol. 15, no. 3, pp. 833–842, 2022, ISSN: 1876-4754.
@article{pmid35636708,
title = {A high-density theta burst paradigm enhances the aftereffects of transcranial magnetic stimulation: Evidence from focal stimulation of rat motor cortex},
author = {Qinglei Meng and Hieu Nguyen and Antonia Vrana and Simone Baldwin and Charlotte Qiong Li and Antonia Giles and Jun Wang and Yihong Yang and Hanbing Lu},
url = {https://pubmed.ncbi.nlm.nih.gov/35636708/},
doi = {10.1016/j.brs.2022.05.017},
issn = {1876-4754},
year = {2022},
date = {2022-05-01},
urldate = {2022-05-01},
journal = {Brain Stimul},
volume = {15},
number = {3},
pages = {833--842},
abstract = {BACKGROUND: Theta burst stimulation (TBS) is an efficient noninvasive neuromodulation paradigm that has been widely adopted, clinically. However, the efficacy of TBS treatment remains similarly modest as conventional 10 Hz repetitive transcranial magnetic stimulation (rTMS).
OBJECTIVE/HYPOTHESIS: To develop a new TBS paradigm that enhances the effects of TMS administration while maintaining high time-efficiency.
METHODS: We describe here a new TMS paradigm, named High-Density Theta Burst Stimulation (hdTBS). This paradigm delivers up to 6 pulses per burst, as opposed to only 3 in conventional TBS, while maintaining the inter-burst interval of 200 ms (or 5 Hz) - a critical parameter in inducing long-term potentiation. This paradigm was implemented on a TMS stimulator developed in-house; its physiological effects were assessed in the motor cortex of awake rats using a rodent specific focal TMS coil. Microwire electrodes were implanted into each rat's limb muscles to longitudinally record motor-evoked potential (MEP). Four different TBS paradigms (3, 4, 5 or 6 pulses per burst, 200 s per session) were tested; MEP signals were recorded immediately before (baseline) and up to 35 min post each TBS session.
RESULTS: We developed a stimulator based on a printed-circuit board strategy. The stimulator was able to deliver stable outputs of up to 6 pulses per burst. Animal experiments (n = 15) revealed significantly different aftereffects induced by the four TBS paradigms (Friedman test, p = 0.018). Post hoc analysis further revealed that, in comparison to conventional 3-pulse TBS, 5- and 6-pulse TBS enhanced the aftereffects of MEP signals by 56% and 92%, respectively, while maintaining identical time efficiency.
CONCLUSION(S): A new stimulation paradigm is proposed, implemented and tested in the motor cortex of awake rats using a focal TMS coil developed in the lab. We observed enhanced aftereffects as assessed by MEP, with no obvious adverse effects, suggesting the translational potentials of this paradigm.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
OBJECTIVE/HYPOTHESIS: To develop a new TBS paradigm that enhances the effects of TMS administration while maintaining high time-efficiency.
METHODS: We describe here a new TMS paradigm, named High-Density Theta Burst Stimulation (hdTBS). This paradigm delivers up to 6 pulses per burst, as opposed to only 3 in conventional TBS, while maintaining the inter-burst interval of 200 ms (or 5 Hz) - a critical parameter in inducing long-term potentiation. This paradigm was implemented on a TMS stimulator developed in-house; its physiological effects were assessed in the motor cortex of awake rats using a rodent specific focal TMS coil. Microwire electrodes were implanted into each rat's limb muscles to longitudinally record motor-evoked potential (MEP). Four different TBS paradigms (3, 4, 5 or 6 pulses per burst, 200 s per session) were tested; MEP signals were recorded immediately before (baseline) and up to 35 min post each TBS session.
RESULTS: We developed a stimulator based on a printed-circuit board strategy. The stimulator was able to deliver stable outputs of up to 6 pulses per burst. Animal experiments (n = 15) revealed significantly different aftereffects induced by the four TBS paradigms (Friedman test, p = 0.018). Post hoc analysis further revealed that, in comparison to conventional 3-pulse TBS, 5- and 6-pulse TBS enhanced the aftereffects of MEP signals by 56% and 92%, respectively, while maintaining identical time efficiency.
CONCLUSION(S): A new stimulation paradigm is proposed, implemented and tested in the motor cortex of awake rats using a focal TMS coil developed in the lab. We observed enhanced aftereffects as assessed by MEP, with no obvious adverse effects, suggesting the translational potentials of this paradigm.
2021
Cover, Christopher G; Kesner, Andrew J; Ukani, Shehzad; Stein, Elliot A; Ikemoto, Satoshi; Yang, Yihong; Lu, Hanbing
Whole brain dynamics during optogenetic self-stimulation of the medial prefrontal cortex in mice Journal Article
In: Communications Biology, vol. 4, no. 1, pp. 66, 2021, ISBN: 2399-3642.
@article{Cover:2021aab,
title = {Whole brain dynamics during optogenetic self-stimulation of the medial prefrontal cortex in mice},
author = {Christopher G Cover and Andrew J Kesner and Shehzad Ukani and Elliot A Stein and Satoshi Ikemoto and Yihong Yang and Hanbing Lu},
url = {https://pubmed.ncbi.nlm.nih.gov/33446857/},
doi = {10.1038/s42003-020-01612-x},
isbn = {2399-3642},
year = {2021},
date = {2021-01-01},
urldate = {2021-01-01},
journal = {Communications Biology},
volume = {4},
number = {1},
pages = {66},
abstract = {Intracranial self-stimulation, in which an animal performs an operant response to receive regional brain electrical stimulation, is a widely used procedure to study motivated behavior. While local neuronal activity has long been measured immediately before or after the operant, imaging the whole brain in real-time remains a challenge. Herein we report a method that permits functional MRI (fMRI) of brain dynamics while mice are cued to perform an operant task: licking a spout to receive optogenetic stimulation to the medial prefrontal cortex (MPFC) during a cue ON, but not cue OFF. Licking during cue ON results in activation of a widely distributed network consistent with underlying MPFC projections, while licking during cue OFF (without optogenetic stimulation) leads to negative fMRI signal in brain regions involved in acute extinction. Noninvasive whole brain readout combined with circuit-specific neuromodulation opens an avenue for investigating adaptive behavior in both healthy and disease models.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
2020
Tsai, Pei-Jung; Keeley, Robin J; Carmack, Stephanie A; Vendruscolo, Janaina C M; Lu, Hanbing; Gu, Hong; Vendruscolo, Leandro F; Koob, George F; Lin, Ching-Po; Stein, Elliot A; Yang, Yihong
Converging structural and functional evidence for a rat salience network Journal Article
In: Biological Psychiatry, 2020, ISBN: 0006-3223.
@article{Tsai:kq,
title = {Converging structural and functional evidence for a rat salience network},
author = {Pei-Jung Tsai and Robin J Keeley and Stephanie A Carmack and Janaina C M Vendruscolo and Hanbing Lu and Hong Gu and Leandro F Vendruscolo and George F Koob and Ching-Po Lin and Elliot A Stein and Yihong Yang},
url = {https://doi.org/10.1016/j.biopsych.2020.06.023},
isbn = {0006-3223},
year = {2020},
date = {2020-06-24},
urldate = {2020-06-24},
booktitle = {Biological Psychiatry},
journal = {Biological Psychiatry},
publisher = {Elsevier},
abstract = {Background
The salience network (SN) is dysregulated in many neuropsychiatric disorders, including substance use disorder. Initially described in humans, identification of a rodent SN would provide the ability to mechanistically interrogate this network in preclinical models of neuropsychiatric disorders.
Methods
We used modularity analysis on resting-state functional MRI data of rats (n=32) to parcellate rat insula into functional subdivisions and to identify a potential rat SN based on functional connectivity patterns from the insular subdivisions. We then used mouse tract tracing data from the Allen brain atlas to confirm the network’s underlying structural connectivity. We next compared functional connectivity profiles of the SN across rat, marmoset (n=10) and humans (n=30). Finally, we assessed rat SN’s response to conditioned cues in rats (n=21) with a history of heroin self-administration.
Results
We identified a putative rat SN, which consists of primarily the ventral anterior insula and anterior cingulate cortex, based on functional connectivity patterns from the ventral anterior insular division. Functional connectivity architecture of the rat SN is supported by the mouse neuronal tracer data. Moreover, the anatomical profile of the identified rat SN is similar to that of non-human primates and humans. Finally, we demonstrate that the rat SN responds to conditioned cues and increases functional connectivity to the Default Mode Network during conditioned heroin withdrawal.
Conclusions
The neurobiological identification of a rat SN together with a demonstration of its functional relevance provides a novel platform with which to interrogate its functional significance in normative and neuropsychiatric disease models.
},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
The salience network (SN) is dysregulated in many neuropsychiatric disorders, including substance use disorder. Initially described in humans, identification of a rodent SN would provide the ability to mechanistically interrogate this network in preclinical models of neuropsychiatric disorders.
Methods
We used modularity analysis on resting-state functional MRI data of rats (n=32) to parcellate rat insula into functional subdivisions and to identify a potential rat SN based on functional connectivity patterns from the insular subdivisions. We then used mouse tract tracing data from the Allen brain atlas to confirm the network’s underlying structural connectivity. We next compared functional connectivity profiles of the SN across rat, marmoset (n=10) and humans (n=30). Finally, we assessed rat SN’s response to conditioned cues in rats (n=21) with a history of heroin self-administration.
Results
We identified a putative rat SN, which consists of primarily the ventral anterior insula and anterior cingulate cortex, based on functional connectivity patterns from the ventral anterior insular division. Functional connectivity architecture of the rat SN is supported by the mouse neuronal tracer data. Moreover, the anatomical profile of the identified rat SN is similar to that of non-human primates and humans. Finally, we demonstrate that the rat SN responds to conditioned cues and increases functional connectivity to the Default Mode Network during conditioned heroin withdrawal.
Conclusions
The neurobiological identification of a rat SN together with a demonstration of its functional relevance provides a novel platform with which to interrogate its functional significance in normative and neuropsychiatric disease models.
Keeley, Robin J; Hsu, Li-Ming; Brynildsen, Julia K; Lu, Hanbing; Yang, Yihong; Stein, Elliot A
In: Neuropsychopharmacology, vol. 45, no. 6, pp. 1042–1049, 2020, ISBN: 1740-634X.
@article{Keeley:2020zj,
title = {Intrinsic differences in insular circuits moderate the negative association between nicotine dependence and cingulate-striatal connectivity strength},
author = {Robin J Keeley and Li-Ming Hsu and Julia K Brynildsen and Hanbing Lu and Yihong Yang and Elliot A Stein},
url = {https://pubmed.ncbi.nlm.nih.gov/32053829/},
doi = {10.1038/s41386-020-0635-x},
isbn = {1740-634X},
year = {2020},
date = {2020-01-01},
journal = {Neuropsychopharmacology},
volume = {45},
number = {6},
pages = {1042--1049},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Cermak, Samantha; Meng, Qinglei; Peng, Kevin; Baldwin, Simone; 'i, Carlos Mej A; Yang, Yihong; Lu, Hanbing
Focal transcranial magnetic stimulation in awake rats: Enhanced glucose uptake in deep cortical layers Journal Article
In: Journal of Neuroscience Methods, vol. 339, pp. 108709, 2020, ISSN: 0165-0270.
@article{CERMAK2020108709,
title = {Focal transcranial magnetic stimulation in awake rats: Enhanced glucose uptake in deep cortical layers},
author = {Samantha Cermak and Qinglei Meng and Kevin Peng and Simone Baldwin and Carlos Mej A 'i and Yihong Yang and Hanbing Lu},
url = {https://pubmed.ncbi.nlm.nih.gov/32259609/},
doi = {https://doi.org/10.1016/j.jneumeth.2020.108709},
issn = {0165-0270},
year = {2020},
date = {2020-01-01},
journal = {Journal of Neuroscience Methods},
volume = {339},
pages = {108709},
abstract = {Background
Transcranial magnetic stimulation (TMS) is an emerging neuromodulation tool. However, preclinical models of TMS are limited.
Objective
To develop a method for performing TMS in awake rats and to characterize neuronal response to TMS by mapping glucose uptake following TMS administration.
Methods
A headpost was implanted into rat skull serving as a refence to guide TMS target. Motor threshold measurement was used as the metric to assess the consistency in TMS delivery across animals and across sessions. Using a fluorescent glucose analogue (2-NBDG) as a marker of neuronal activity, we mapped glucose uptake in response to TMS of the rat motor cortex.
Results The average motor threshold (n = 41) was 34.6 $pm$ 6.3 % of maximum stimulator output (MSO). The variability of motor threshold across animals was similar to what has been reported in human studies. Furthermore, there was no significant difference in motor threshold measured across 3 separate days. Enhancement in fluorescent signals were TMS dose (power)-dependent, which centered around the motor cortex, covering an area medial-laterally 2 mm, rostral-caudally 4 mm at 55 % MSO, and 3 mm at 35 % MSO. The count of total cells with significant fluorescent signal was: 107 $pm$ 23 (55 % MSO), 73 $pm$ 11 (35 % MSO) and 42 $pm$ 11 (sham, 5% MSO).
Conclusions
Our method allows for consistent motor threshold assessment for longitudinal studies. Notably, cells with fluorescent signal enhancement were consistently aggregated in deep cortical layers, with minimal enhancement in superficial layers
Comparisons with existing method(s)
To our knowledge, this is the first study of focal TMS in awake rodents.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Transcranial magnetic stimulation (TMS) is an emerging neuromodulation tool. However, preclinical models of TMS are limited.
Objective
To develop a method for performing TMS in awake rats and to characterize neuronal response to TMS by mapping glucose uptake following TMS administration.
Methods
A headpost was implanted into rat skull serving as a refence to guide TMS target. Motor threshold measurement was used as the metric to assess the consistency in TMS delivery across animals and across sessions. Using a fluorescent glucose analogue (2-NBDG) as a marker of neuronal activity, we mapped glucose uptake in response to TMS of the rat motor cortex.
Results The average motor threshold (n = 41) was 34.6 $pm$ 6.3 % of maximum stimulator output (MSO). The variability of motor threshold across animals was similar to what has been reported in human studies. Furthermore, there was no significant difference in motor threshold measured across 3 separate days. Enhancement in fluorescent signals were TMS dose (power)-dependent, which centered around the motor cortex, covering an area medial-laterally 2 mm, rostral-caudally 4 mm at 55 % MSO, and 3 mm at 35 % MSO. The count of total cells with significant fluorescent signal was: 107 $pm$ 23 (55 % MSO), 73 $pm$ 11 (35 % MSO) and 42 $pm$ 11 (sham, 5% MSO).
Conclusions
Our method allows for consistent motor threshold assessment for longitudinal studies. Notably, cells with fluorescent signal enhancement were consistently aggregated in deep cortical layers, with minimal enhancement in superficial layers
Comparisons with existing method(s)
To our knowledge, this is the first study of focal TMS in awake rodents.
2019
Hsu, Li-Ming; Keeley, Robin J; Liang, Xia; Brynildsen, Julia K; Lu, Hanbing; Yang, Yihong; Stein, Elliot A
Intrinsic Insular-Frontal Networks Predict Future Nicotine Dependence Severity. Journal Article
In: J Neurosci, vol. 39, no. 25, pp. 5028–5037, 2019, ISSN: 1529-2401 (Electronic); 0270-6474 (Linking).
@article{Hsu:2019aa,
title = {Intrinsic Insular-Frontal Networks Predict Future Nicotine Dependence Severity.},
author = {Li-Ming Hsu and Robin J Keeley and Xia Liang and Julia K Brynildsen and Hanbing Lu and Yihong Yang and Elliot A Stein},
url = {https://www.ncbi.nlm.nih.gov/pubmed/30992371},
doi = {10.1523/JNEUROSCI.0140-19.2019},
issn = {1529-2401 (Electronic); 0270-6474 (Linking)},
year = {2019},
date = {2019-06-19},
journal = {J Neurosci},
volume = {39},
number = {25},
pages = {5028--5037},
address = {Neuroimaging Research Branch, National Institute on Drug Abuse, Intramural Research Program, Baltimore, Maryland 21224, and.},
abstract = {Although 60% of the US population have tried smoking cigarettes, only 16% smoke regularly. Identifying this susceptible subset of the population before the onset of nicotine dependence may encourage targeted early interventions to prevent regular smoking and/or minimize severity. While prospective neuroimaging in human populations can be challenging, preclinical neuroimaging models before chronic nicotine administration can help to develop translational biomarkers of disease risk. Chronic, intermittent nicotine (0, 1.2, or 4.8 mg/kg/d; N = 10-11/group) was administered to male Sprague Dawley rats for 14 d; dependence severity was quantified using precipitated withdrawal behaviors collected before, during, and following forced nicotine abstinence. Resting-state fMRI functional connectivity (FC) before drug administration was subjected to a graph theory analytical framework to form a predictive model of subsequent individual differences in nicotine dependence. Whole-brain modularity analysis identified five modules in the rat brain. A metric of intermodule connectivity, participation coefficient, of an identified insular-frontal cortical module predicted subsequent dependence severity, independent of nicotine dose. To better spatially isolate this effect, this module was subjected to a secondary exploratory modularity analysis, which segregated it into three submodules (frontal-motor, insular, and sensory). Higher FC among these three submodules and three of the five originally identified modules (striatal, frontal-executive, and sensory association) also predicted dependence severity. These data suggest that predispositional, intrinsic differences in circuit strength between insular-frontal-based brain networks before drug exposure may identify those at highest risk for the development of nicotine dependence.SIGNIFICANCE STATEMENT Developing biomarkers of individuals at high risk for addiction before the onset of this brain-based disease is essential for prevention, early intervention, and/or subsequent treatment decisions. Using a rodent model of nicotine dependence and a novel data-driven, network-based analysis of resting-state fMRI data collected before drug exposure, functional connections centered on an intrinsic insular-frontal module predicted the severity of nicotine dependence after drug exposure. The predictive capacity of baseline network measures was specific to inter-regional but not within-region connectivity. While insular and frontal regions have consistently been implicated in nicotine dependence, this is the first study to reveal that innate, individual differences in their circuit strength have the predictive capacity to identify those at greatest risk for and resilience to drug dependence.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Hu, Yuzheng; Salmeron, Betty Jo; Krasnova, Irina N; Gu, Hong; Lu, Hanbing; Bonci, Antonello; Cadet, Jean L; Stein, Elliot A; Yang, Yihong
Compulsive drug use is associated with imbalance of orbitofrontal- and prelimbic-striatal circuits in punishment-resistant individuals. Journal Article
In: Proc Natl Acad Sci U S A, vol. 116, no. 18, pp. 9066–9071, 2019, ISSN: 1091-6490 (Electronic); 0027-8424 (Linking).
@article{Hu:2019aa,
title = {Compulsive drug use is associated with imbalance of orbitofrontal- and prelimbic-striatal circuits in punishment-resistant individuals.},
author = {Yuzheng Hu and Betty Jo Salmeron and Irina N Krasnova and Hong Gu and Hanbing Lu and Antonello Bonci and Jean L Cadet and Elliot A Stein and Yihong Yang},
url = {https://www.ncbi.nlm.nih.gov/pubmed/30988198},
doi = {10.1073/pnas.1819978116},
issn = {1091-6490 (Electronic); 0027-8424 (Linking)},
year = {2019},
date = {2019-04-30},
urldate = {2019-04-30},
journal = {Proc Natl Acad Sci U S A},
volume = {116},
number = {18},
pages = {9066--9071},
address = {Neuroimaging Research Branch, National Institute on Drug Abuse, Intramural Research Programs, National Institutes of Health, Baltimore, MD 21224; yihongyang@intra.nida.nih.gov huyuzheng@zju.edu.cn.},
abstract = {Substance use disorders (SUDs) impose severe negative impacts upon individuals, their families, and society. Clinical studies demonstrate that some chronic stimulant users are able to curtail their drug use when faced with adverse consequences while others continue to compulsively use drugs. The mechanisms underlying this dichotomy are poorly understood, which hampers the development of effective individualized treatments of a disorder that currently has no Food and Drug Administration-approved pharmacological treatments. In the present study, using a rat model of methamphetamine self-administration (SA) in the presence of concomitant foot shocks, thought to parallel compulsive drug taking by humans, we found that SA behavior correlated with alterations in the balance between an increased orbitofrontal cortex-dorsomedial striatal "go" circuit and a decreased prelimbic cortex-ventrolateral striatal "stop" circuit. Critically, this correlation was seen only in rats who continued to self-administer at a relatively high rate despite receiving foot shocks of increasing intensity. While the stop circuit functional connectivity became negative after repeated SA in all rats, "shock-resistant" rats showed strengthening of this negative connectivity after shock exposure. In contrast, "shock-sensitive" rats showed a return toward their baseline levels after shock exposure. These results may help guide novel noninvasive brain stimulation therapies aimed at restoring the physiological balance between stop and go circuits in SUDs.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Jaime, Saul; Gu, Hong; Sadacca, Brian F; Stein, Elliot A; Cavazos, Jose E; Yang, Yihong; Lu, Hanbing
Delta Rhythm Orchestrates the Neural Activity Underlying the Resting State BOLD Signal via Phase-amplitude Coupling. Journal Article
In: Cereb Cortex, pp. 1–15, 2019, ISSN: 1460-2199 (Electronic); 1047-3211 (Linking).
@article{Jaime:2017aa,
title = {Delta Rhythm Orchestrates the Neural Activity Underlying the Resting State BOLD Signal via Phase-amplitude Coupling.},
author = {Saul Jaime and Hong Gu and Brian F Sadacca and Elliot A Stein and Jose E Cavazos and Yihong Yang and Hanbing Lu},
url = {https://www.ncbi.nlm.nih.gov/pubmed/29161352},
doi = {10.1093/cercor/bhx310},
issn = {1460-2199 (Electronic); 1047-3211 (Linking)},
year = {2019},
date = {2019-01-01},
journal = {Cereb Cortex},
pages = {1--15},
address = {Neuroimaging Research Branch, National Institute on Drug Abuse, National Institutes of Health, Baltimore, USA.},
abstract = {Spontaneous ongoing neuronal activity is a prominent feature of the mammalian brain. Temporal and spatial patterns of such ongoing activity have been exploited to examine large-scale brain network organization and function. However, the neurophysiological basis of this spontaneous brain activity as detected by resting-state functional Magnetic Resonance Imaging (fMRI) remains poorly understood. To this end, multi-site local field potentials (LFP) and blood oxygenation level-dependent (BOLD) fMRI were simultaneously recorded in the rat striatum along with local pharmacological manipulation of striatal activity. Results demonstrate that delta (delta) band LFP power negatively, while beta (beta) and gamma (gamma) band LFPs positively correlated with BOLD fluctuation. Furthermore, there was strong cross-frequency phase-amplitude coupling (PAC), with the phase of delta LFPs significantly modulating the amplitude of the high frequency signal. Enhancing dopaminergic neuronal activity significantly reduced ventral striatal functional connectivity, delta LFP-BOLD correlation, and the PAC effect. These data suggest that different frequency bands of the LFP contribute distinctively to BOLD spontaneous fluctuation and that PAC is the organizing mechanism through which low frequency LFPs orchestrate neural activity that underlies resting state functional connectivity.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Sumiyoshi, Akira; Keeley, Robin J; Lu, Hanbing
Physiological Considerations of Functional Magnetic Resonance Imaging in Animal Models Journal Article
In: Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, vol. 4, no. 6, pp. 522-532, 2019, ISSN: 2451-9022, (The Bridging of Scales: Techniques for Translational Neuroscience).
@article{SUMIYOSHI2019522,
title = {Physiological Considerations of Functional Magnetic Resonance Imaging in Animal Models},
author = {Akira Sumiyoshi and Robin J Keeley and Hanbing Lu},
url = {https://pubmed.ncbi.nlm.nih.gov/30270095/},
doi = {https://doi.org/10.1016/j.bpsc.2018.08.002},
issn = {2451-9022},
year = {2019},
date = {2019-01-01},
journal = {Biological Psychiatry: Cognitive Neuroscience and Neuroimaging},
volume = {4},
number = {6},
pages = {522-532},
abstract = {Characterizing the nature and underlying neurobiological causes of psychiatric and neurological diseases at the circuit and network levels has remained elusive and necessitates the use of robust animal models. Noninvasive functional magnetic resonance imaging allows systems-level insight into disease phenotype in humans and animals alike, and functional neuroimaging represents an ideal platform for translational and reverse-translational research, with common measurements collected across species. Animal neuroimaging allows invasive manipulations and conveniently bypasses many limitations associated with human subjects; however, awake animal imaging introduces its own constraints to reduce motion and limit subjective stress. Anesthetics offer a viable alternative, but the pharmacodynamics, pharmacokinetics, and molecular targets of anesthetics and their effects on physiology, neural activity, and neurovascular coupling must be considered. We discuss the physiological basis of and the influence of anesthetics on neurovascular coupling. We discuss anesthetic use in functional magnetic resonance imaging and focus on an anesthetic protocol developed in our laboratory. Finally, we discuss in detail our most recent work examining the physiological basis of resting-state functional magnetic resonance imaging using this anesthetic regimen and the future directions of animal neuroimaging research. Using animal imaging in combination with cutting-edge in vivo neuromodulatory techniques is essential for causal understanding of brain function in health and disease and offers an exemplary bridge between human and animal research studies.},
note = {The Bridging of Scales: Techniques for Translational Neuroscience},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
2018
Meng, Qinglei; Jing, Li; Badjo, Jean Paul; Du, Xiaoming; Hong, Elliot; Yang, Yihong; Lu, Hanbing; Choa, Fow-Sen
A novel transcranial magnetic stimulator for focal stimulation of rodent brain. Journal Article
In: Brain Stimul, vol. 11, no. 3, pp. 663–665, 2018, ISSN: 1876-4754 (Electronic); 1876-4754 (Linking).
@article{Meng:2018aa,
title = {A novel transcranial magnetic stimulator for focal stimulation of rodent brain.},
author = {Qinglei Meng and Li Jing and Jean Paul Badjo and Xiaoming Du and Elliot Hong and Yihong Yang and Hanbing Lu and Fow-Sen Choa},
url = {https://www.ncbi.nlm.nih.gov/pubmed/29534946},
doi = {10.1016/j.brs.2018.02.018},
issn = {1876-4754 (Electronic); 1876-4754 (Linking)},
year = {2018},
date = {2018-06-01},
urldate = {2018-06-01},
journal = {Brain Stimul},
volume = {11},
number = {3},
pages = {663--665},
address = {Department of Computer Science and Electrical Engineering, University of Maryland, Baltimore County, MD, United States; Intramural Research Program, National Institute on Drug Abuse (NIDA), National Institutes of Health (NIH), Baltimore, MD, United States.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
2016
Ash, Jessica A; Lu, Hanbing; Taxier, Lisa R; Long, Jeffrey M; Yang, Yihong; Stein, Elliot A; Rapp, Peter R
Functional connectivity with the retrosplenial cortex predicts cognitive aging in rats Journal Article
In: Proceedings of the National Academy of Sciences, vol. 113, no. 43, pp. 12286–12291, 2016, ISSN: 0027-8424.
@article{Ash12286,
title = {Functional connectivity with the retrosplenial cortex predicts cognitive aging in rats},
author = {Jessica A Ash and Hanbing Lu and Lisa R Taxier and Jeffrey M Long and Yihong Yang and Elliot A Stein and Peter R Rapp},
url = {https://pubmed.ncbi.nlm.nih.gov/27791017/},
doi = {10.1073/pnas.1525309113},
issn = {0027-8424},
year = {2016},
date = {2016-01-01},
journal = {Proceedings of the National Academy of Sciences},
volume = {113},
number = {43},
pages = {12286--12291},
publisher = {National Academy of Sciences},
abstract = {Neural network dynamics thought to play a key role in cognition are substantially disrupted in both normal and pathological aging. Using a rat model, here we aimed to define the effects of aging on the integrity of cortical resting state functional connectivity distinct from the potential contribution of neurodegenerative disease. The findings highlight that disrupted circuit connectivity with the retrosplenial/posterior cingulate cortex is coupled with variability in memory function during aging, and that adaptive plasticity in the aged brain appears to contribute to successful cognitive aging. The development of interventions that promote neuroadaptive network plasticity is a potentially valuable alternative to strategies currently under investigation, toward bending the trajectory of aging away from neurodegeneration.Changes in the functional connectivity (FC) of large-scale brain networks are a prominent feature of brain aging, but defining their relationship to variability along the continuum of normal and pathological cognitive outcomes has proved challenging. Here we took advantage of a well-characterized rat model that displays substantial individual differences in hippocampal memory during aging, uncontaminated by slowly progressive, spontaneous neurodegenerative disease. By this approach, we aimed to interrogate the underlying neural network substrates that mediate aging as a uniquely permissive condition and the primary risk for neurodegeneration. Using resting state (rs) blood oxygenation level-dependent fMRI and a restrosplenial/posterior cingulate cortex seed, aged rats demonstrated a large-scale network that had a spatial distribution similar to the default mode network (DMN) in humans, consistent with earlier findings in younger animals. Between-group whole brain contrasts revealed that aged subjects with documented deficits in memory (aged impaired) displayed widespread reductions in cortical FC, prominently including many areas outside the DMN, relative to both young adults (Y) and aged rats with preserved memory (aged unimpaired, AU). Whereas functional connectivity was relatively preserved in AU rats, they exhibited a qualitatively distinct network signature, comprising the loss of an anticorrelated network observed in Y adults. Together the findings demonstrate that changes in rs-FC are specifically coupled to variability in the cognitive outcome of aging, and that successful neurocognitive aging is associated with adaptive remodeling, not simply the persistence of youthful network dynamics.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
2014
Lu, Hanbing; Wang, Leiming; Rea, William W; Brynildsen, Julia K; Jaime, Saul; Zuo, Yantao; Stein, Elliot A; Yang, Yihong
Low- but Not High-Frequency LFP Correlates with Spontaneous BOLD Fluctuations in Rat Whisker Barrel Cortex. Journal Article
In: Cereb Cortex, vol. 26, no. 2, pp. 683–694, 2014, ISSN: 1460-2199 (Electronic); 1047-3211 (Linking).
@article{Lu2014,
title = {Low- but Not High-Frequency LFP Correlates with Spontaneous BOLD Fluctuations in Rat Whisker Barrel Cortex.},
author = {Hanbing Lu and Leiming Wang and William W Rea and Julia K Brynildsen and Saul Jaime and Yantao Zuo and Elliot A Stein and Yihong Yang},
url = {https://www.ncbi.nlm.nih.gov/pubmed/25331598},
doi = {10.1093/cercor/bhu248},
issn = {1460-2199 (Electronic); 1047-3211 (Linking)},
year = {2014},
date = {2014-10-20},
journal = {Cereb Cortex},
volume = {26},
number = {2},
pages = {683--694},
address = {Neuroimaging Research Branch and.},
abstract = {Resting-state magnetic resonance imaging (rsMRI) is thought to reflect ongoing spontaneous brain activity. However, the precise neurophysiological basis of rsMRI signal remains elusive. Converging evidence supports the notion that local field potential (LFP) signal in the high-frequency range correlates with fMRI response evoked by a task (e.g., visual stimulation). It remains uncertain whether this relationship extends to rsMRI. In this study, we systematically modulated LFP signal in the whisker barrel cortex (WBC) by unilateral deflection of rat whiskers. Results show that functional connectivity between bilateral WBC was significantly modulated at the 2 Hz, but not at the 4 or 6 Hz, stimulus condition. Electrophysiologically, only in the low-frequency range (<5 Hz) was the LFP power synchrony in bilateral WBC significantly modulated at 2 Hz, but not at 4- or 6-Hz whisker stimulation, thus distinguishing these 2 experimental conditions, and paralleling the findings in rsMRI. LFP power synchrony in other frequency ranges was modulated in a way that was neither unique to the specific stimulus conditions nor parallel to the fMRI results. Our results support the hypothesis that emphasizes the role of low-frequency LFP signal underlying rsMRI.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
2012
Lu, Hanbing; Zou, Qihong; Gu, Hong; Raichle, Marcus E; Stein, Elliot A; Yang, Yihong
Rat brains also have a default mode network. Journal Article
In: Proc Natl Acad Sci U S A, vol. 109, no. 10, pp. 3979–3984, 2012, ISSN: 1091-6490 (Electronic); 0027-8424 (Linking).
@article{Lu2012,
title = {Rat brains also have a default mode network.},
author = {Hanbing Lu and Qihong Zou and Hong Gu and Marcus E Raichle and Elliot A Stein and Yihong Yang},
url = {https://www.ncbi.nlm.nih.gov/pubmed/22355129},
doi = {10.1073/pnas.1200506109},
issn = {1091-6490 (Electronic); 0027-8424 (Linking)},
year = {2012},
date = {2012-03-06},
journal = {Proc Natl Acad Sci U S A},
volume = {109},
number = {10},
pages = {3979--3984},
address = {Neuroimaging Research Branch, National Institute on Drug Abuse, Intramural Research Programs, National Institutes of Health, Baltimore, MD 21224, USA.},
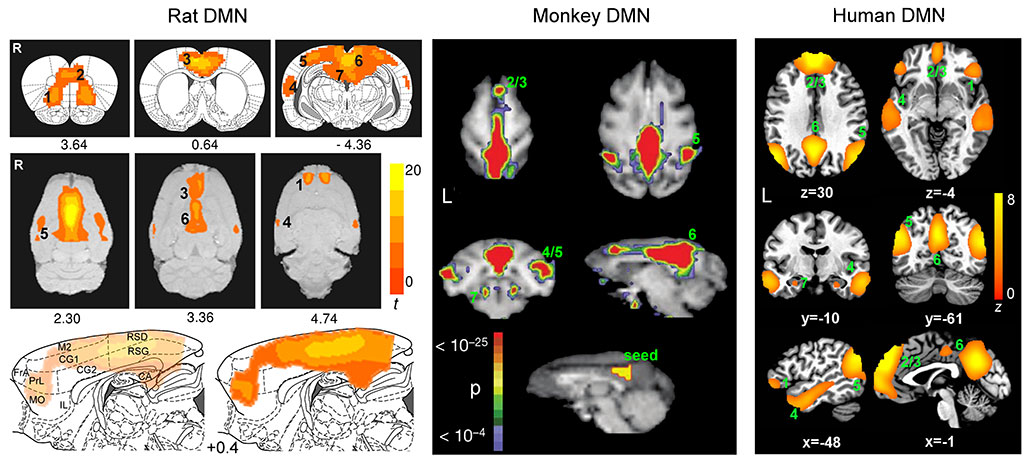
abstract = {The default mode network (DMN) in humans has been suggested to support a variety of cognitive functions and has been implicated in an array of neuropsychological disorders. However, its function(s) remains poorly understood. We show that rats possess a DMN that is broadly similar to the DMNs of nonhuman primates and humans. Our data suggest that, despite the distinct evolutionary paths between rodent and primate brain, a well-organized, intrinsically coherent DMN appears to be a fundamental feature in the mammalian brain whose primary functions might be to integrate multimodal sensory and affective information to guide behavior in anticipation of changing environmental contingencies.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
2007
Lu, Hanbing; Zuo, Yantao; Gu, Hong; Waltz, James A; Zhan, Wang; Scholl, Clara A; Rea, William; Yang, Yihong; Stein, Elliot A
Synchronized delta oscillations correlate with the resting-state functional MRI signal. Journal Article
In: Proc Natl Acad Sci U S A, vol. 104, no. 46, pp. 18265–18269, 2007, ISSN: 1091-6490 (Electronic); 0027-8424 (Linking).
@article{Lu2007,
title = {Synchronized delta oscillations correlate with the resting-state functional MRI signal.},
author = {Hanbing Lu and Yantao Zuo and Hong Gu and James A Waltz and Wang Zhan and Clara A Scholl and William Rea and Yihong Yang and Elliot A Stein},
url = {https://www.ncbi.nlm.nih.gov/pubmed/17991778},
doi = {10.1073/pnas.0705791104},
issn = {1091-6490 (Electronic); 0027-8424 (Linking)},
year = {2007},
date = {2007-11-08},
journal = {Proc Natl Acad Sci U S A},
volume = {104},
number = {46},
pages = {18265--18269},
address = {Neuroimaging Research Branch, National Institute on Drug Abuse, Intramural Research Program, National Institutes of Health, Baltimore, MD 21224, USA.},
abstract = {Synchronized low-frequency spontaneous fluctuations of the functional MRI (fMRI) signal have recently been applied to investigate large-scale neuronal networks of the brain in the absence of specific task instructions. However, the underlying neural mechanisms of these fluctuations remain largely unknown. To this end, electrophysiological recordings and resting-state fMRI measurements were conducted in alpha-chloralose-anesthetized rats. Using a seed-voxel analysis strategy, region-specific, anesthetic dose-dependent fMRI resting-state functional connectivity was detected in bilateral primary somatosensory cortex (S1FL) of the resting brain. Cortical electroencephalographic signals were also recorded from bilateral S1FL; a visual cortex locus served as a control site. Results demonstrate that, unlike the evoked fMRI response that correlates with power changes in the gamma bands, the resting-state fMRI signal correlates with the power coherence in low-frequency bands, particularly the delta band. These data indicate that hemodynamic fMRI signal differentially registers specific electrical oscillatory frequency band activity, suggesting that fMRI may be able to distinguish the ongoing from the evoked activity of the brain.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Lu, Hanbing; Xi, Zheng-Xiong; Gitajn, Leah; Rea, William; Yang, Yihong; Stein, Elliot A
Cocaine-induced brain activation detected by dynamic manganese-enhanced magnetic resonance imaging (MEMRI). Journal Article
In: Proc Natl Acad Sci U S A, vol. 104, no. 7, pp. 2489–2494, 2007, ISSN: 0027-8424 (Print); 0027-8424 (Linking).
@article{Lu2007c,
title = {Cocaine-induced brain activation detected by dynamic manganese-enhanced magnetic resonance imaging (MEMRI).},
author = {Hanbing Lu and Zheng-Xiong Xi and Leah Gitajn and William Rea and Yihong Yang and Elliot A Stein},
url = {https://www.ncbi.nlm.nih.gov/pubmed/17287361},
doi = {10.1073/pnas.0606983104},
issn = {0027-8424 (Print); 0027-8424 (Linking)},
year = {2007},
date = {2007-02-07},
journal = {Proc Natl Acad Sci U S A},
volume = {104},
number = {7},
pages = {2489--2494},
address = {Neuroimaging Research Branch, National Institute on Drug Abuse (NIDA), Intramural Research Program, National Institutes of Health, Baltimore, MD 21224, USA.},
abstract = {Dynamic manganese-enhanced magnetic resonance imaging (MEMRI) detects neuronal activity based on the passage of Mn(2+) into active neurons. Because this mechanism is independent of any hemodynamic response, it is potentially ideal for pharmacological studies and was applied to investigate the acute CNS effects of cocaine in the rat. Dose-dependent, region-specific MEMRI signals were seen mostly in cortical and subcortical mesocorticolimbic structures. To verify the spatial accuracy and physiological mechanisms of MEMRI, neuronal activation following electrical forepaw stimulation revealed somatotopic signal enhancement in the primary and secondary somatosensory cortices, which was blocked by diltiazem, a Ca2+ channel antagonist. These data suggest that MEMRI may serve as a tool for investigating the effects of pharmacological agents and opens an application of MRI to study CNS drug effects at a systems level.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
2004
Lu, Hanbing; Patel, Sachin; Luo, Feng; Li, Shi-Jiang; Hillard, Cecilia J; Ward, Douglas B; Hyde, James S
Spatial correlations of laminar BOLD and CBV responses to rat whisker stimulation with neuronal activity localized by Fos expression. Journal Article
In: Magn Reson Med, vol. 52, no. 5, pp. 1060–1068, 2004, ISSN: 0740-3194 (Print); 0740-3194 (Linking).
@article{Lu2004,
title = {Spatial correlations of laminar BOLD and CBV responses to rat whisker stimulation with neuronal activity localized by Fos expression.},
author = {Hanbing Lu and Sachin Patel and Feng Luo and Shi-Jiang Li and Cecilia J Hillard and Douglas B Ward and James S Hyde},
url = {https://www.ncbi.nlm.nih.gov/pubmed/15508149},
doi = {10.1002/mrm.20265},
issn = {0740-3194 (Print); 0740-3194 (Linking)},
year = {2004},
date = {2004-11-01},
journal = {Magn Reson Med},
volume = {52},
number = {5},
pages = {1060--1068},
address = {Department of Biophysics, Medical College of Wisconsin, Milwaukee 53226, USA.},
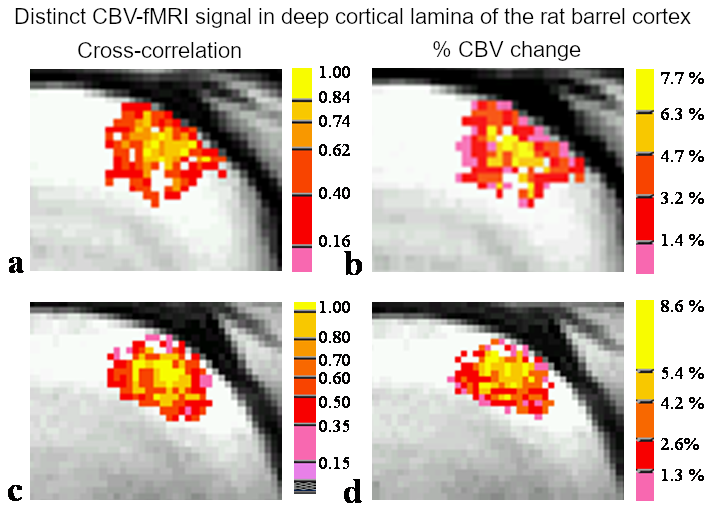
abstract = {The spatial relationship between a measured fMRI signal and its underlying neuronal activity remains unclear. One obstacle is the localization of neuronal activity; another is the spatial resolution of fMRI. In the present study, high-resolution BOLD and CBV fMRI experiments (voxel size: 156 x 156 x 2000 microm3) were conducted in the rat whisker barrel cortex at 3 T; neuronal activity across cortical layers was mapped using the Fos expression technique. Results show that BOLD response is weighted by blood volume and that pixels with high BOLD response can be located at the cortical surface or in deep layers, depending on local vasculature. In contrast to BOLD response, the pixels with high CBV response were consistently clustered in the deep cortical layers. Percentage-CBV change in cortical layers IV-V was 7.3 +/- 1.5%, which was significantly higher than in layers I-III (4.1 +/- 0.9%) and VI (4.3 +/- 0.7%) (mean +/- SEM). The laminar distribution of CBV response correlates well with neuronal activity localized by Fos expression. We conclude that neuronal activity can be inferred from CBV fMRI data with high spatial accuracy. The data indicate that both intracolumn functional connectivity and neurovascular coupling can be studied using CBV fMRI.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}