Background | Status & Availability | Transgene Info | Phenotypic Characterization | Breeding | Genotyping | References | Blog/Comments/Reviews | Related rats | Acknowledgements
Background
Genetically-encoded fluorescent reporters are used in the biological sciences for the identification and observation of cells that express transgenic proteins. Near-infrared (NIR) fluorescent proteins have superior light penetration through biological tissue, but are not yet widely adopted. Using the near-infrared fluorescent protein, iRFP713, improves the imaging resolution in thick tissue sections or the intact brain due to the reduced light-scattering at the longer, NIR wavelengths used to image the protein. Additionally, iRFP713 can be used to identify transgenic cells without photobleaching other fluorescent reporters or affecting opsin function for optogenetic experiments. We have generated a transgenic rat (Sprague Dawley background) that expresses iRFP713 in a Cre-dependent manner.
Status and Availability
This rat has been published (PMID: 28380331).
As of May 30, 2017, this strain is available as
This strain is available as line #748 at the RRRC. ![]()
This rat is registered at the Rat Genome Database (RGD) as RGD ID#11081153. ![]()
Transgene Information
Figure 1. Schematic of the DIO-iRFP transgene. The insert encoding iRFP713 was amplified with linkered oligos and Addgene #31857 as a template. This insert was recombined into the backbone (pAAV EF1a DIO EYFP, Addgene 27056, digested with NheI and AscI restriction enzymes) using In-Fusion cloning mix (Clontech) to produce pOTTC374 (Addgene #47626). The pOTTC374 (pAAV EF1a DIO iRFP) plasmid was digested with MluI and RsrII, gel purified away from the plasmid backbone, and microinjected into fertilized oocytes harvested from a Sprague-Dawly rat by the NIMH Transgenic Core. Surviving pups were screened for the integrated transgene by PCR genotyping. This line (SD-Tg(DIO-iRFP)9Ottc) has 3 copies of the transgene per copy of Ggt1 (# copy per haploid genome) as determined by droplet digital PCR.
Phenotypic Characterization
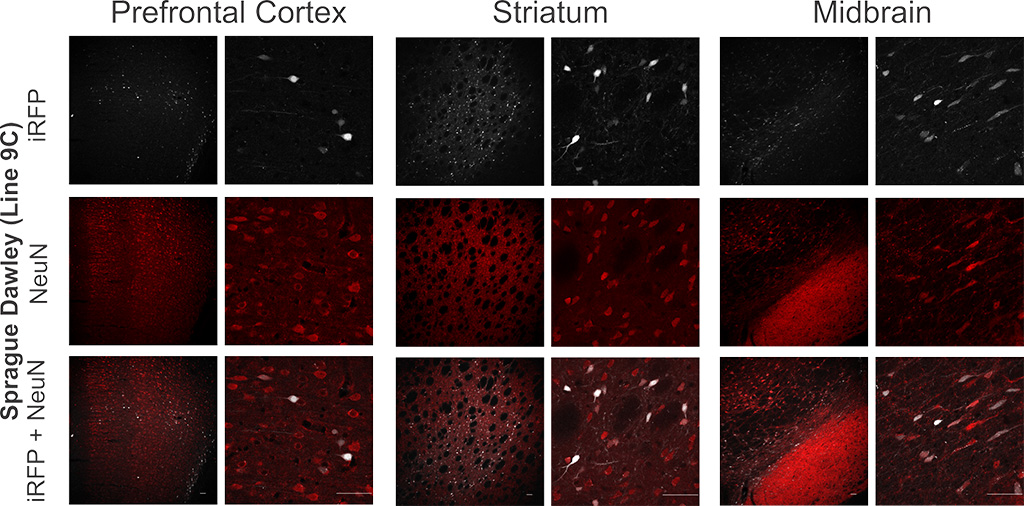
Figure 2. DIO-iRFP transgenic rats express iRFP713 in a Cre-dependent manner in three different brain regions. Representative images of the iRFP expression in phenotypically positive Sprague-Dawley DIO-iRFP transgenic rats. SD-Tg(DIO-iRFP)9Ottc rats were injected in the prefrontal cortex (left), striatum (middle), and midbrain (right) with AAV1-GFP-Cre. Immunohistochemistry for NeuN was used to indicate neurons, which colocalized with iRFP expression. Scale bars are 50 μm. Download print resolution version here.
Figure 2 was modified and reprinted from “Richie et al. Near-infrared fluorescent protein iRFP713 as a reporter protein for optogenetic vectors, a transgenic Cre-reporter rat, and other neuronal studies. J Neurosci Methods. 2017 Jun 1;284:1-14.” With permission from Elsevier.
For more details and data on phenotypic characterization, see reference (PMID: 28380331).
Breeding Strategy
Breeding Information, click here for PDF
Genotyping Assays
Assay for presence of EF1a and iRFP, click here for PDF
References that cite this rat
2017
In: J Neurosci Methods, vol. 284, pp. 1–14, 2017, ISSN: 1872-678X (Electronic); 0165-0270 (Linking), (*First paper describing LE-Tg (DIO-iRFP)3Ottc rat. *First paper describing LE-Tg (DIO-iRFP)9Ottc rat.).
Blog/Comments/Reviews
Last Updated on November 12, 2024
There are 2 surveyed reports for the receiving and usage of the Sprague Dawley transgenic DIO-iRFP rat and only expression issues were reported with the transgenic rat.
General Health
There are no reports of general health issues with the SD-Tg(DIO-iRFP)9Ottc rats
Weight
There are no reports of weight changes with the SD-Tg(DIO-iRFP)9Ottc rats
Breeding
There are no reports of breeding changes with the SD-Tg(DIO-iRFP)9Ottc rats
Expression
One lab crossed the DIO-iRFP with ORX-Cre transgenic rats. Their analysis showed Cre expression outside of the lateral hypothalamus in the brain.
Other related rats
Registered Symbol: LE-Tg(DIO-iRFP)3Ottc
RGD ID: 9588559 ![]()
Proposed Name: LE-Tg(DIO-iRFP)3Ottc
RRRC# 747 ![]()
*Rat Research and Resource Center (RRRC)
Acknowledgements
YaJun Zhang, Julie Necarsulmer, Chris Richie, Brandon Harvey, Janette Lebron