Background | Status & Availability | Transgene Info | Phenotypic Characterization | Breeding | Genotyping | References | Blog/Comments/Reviews | Related rats | Acknowledgements
Background
Status and Availability
This strain has been published (PMID: 31818977).
As of May 17, 2016, this strain is available as line #00773 at the RRRC.
This rat is registered at the Rat Genome Database (RGD) as RGD ID# 10412329.
Transgene Information
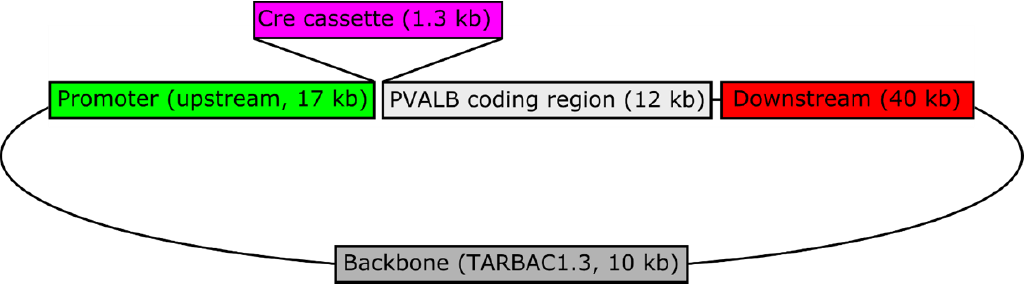
Figure 1: Schematic of Pvalb-iCre transgenic construct. A bacterial artificial chromosome (BAC) containing the rat PVALB gene (CH230-499N20) was obtained from the Children’s Hospital Oakland Research Institute (CHORI), and recombineered to replace the start codon of PVALB with cassette containing iCre (improved Cre recombinase) and the polyadenylation signal from the gene for bovine growth hormone (pOTTC465). This BAC was injected into the pronuclei of fertilized Long Evans rat embryos by NIMH Transgenic Core, and ultimately resulted in three independent, phenotypically positive PVALB::iCre lines. This line (LE-Tg(PVALB -iCre)2Ottc) has one copy of the transgene per haploid genome as determined by droplet digital PCR.
Phenotypic Characterization
Breeding Strategy
Breeding Information, click here for PDF
Genotyping Assays
Assay for presence of Pvalb and iCre, click here for PDF
References that cite this rat
2022
Miranda, Janelle M; Cruz, Emmanuel; Bessières, Benjamin; Alberini, Cristina M
Hippocampal parvalbumin interneurons play a critical role in memory development Journal Article
In: Cell Rep, vol. 41, no. 7, pp. 111643, 2022, ISSN: 2211-1247.
@article{pmid36384113,
title = {Hippocampal parvalbumin interneurons play a critical role in memory development},
author = {Janelle M Miranda and Emmanuel Cruz and Benjamin Bessières and Cristina M Alberini},
url = {https://pubmed.ncbi.nlm.nih.gov/36384113/},
doi = {10.1016/j.celrep.2022.111643},
issn = {2211-1247},
year = {2022},
date = {2022-11-01},
urldate = {2022-11-01},
journal = {Cell Rep},
volume = {41},
number = {7},
pages = {111643},
abstract = {Episodic memories formed in early childhood rapidly decay, but their latent traces remain stored long term. These memories require the dorsal hippocampus (dHPC) and seem to undergo a developmental critical period. It remains to be determined whether the maturation of parvalbumin interneurons (PVIs), a major mechanism of critical periods, contributes to memory development. Here, we show that episodic infantile learning significantly increases the levels of parvalbumin in the dHPC 48 h after training. Chemogenetic inhibition of PVIs before learning indicated that these neurons are required for infantile memory formation. A bilateral dHPC injection of the γ-aminobutyric acid type A receptor agonist diazepam after training elicited long-term memory expression in infant rats, although direct PVI chemogenetic activation had no effect. Finally, PVI activity was required for brain-derived neurotrophic factor (BDNF)-dependent maturation of memory competence, i.e., adult-like long-term memory expression. Thus, dHPC PVIs are critical for the formation of infantile memories and for memory development.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Armenta-Resendiz, Monserrat; Assali, Ahlem; Tsvetkov, Evgeny; Cowan, Christopher W; Lavin, Antonieta
In: Neuropsychopharmacology, vol. 47, no. 10, pp. 1816–1825, 2022, ISSN: 1740-634X.
@article{pmid35788684,
title = {Repeated methamphetamine administration produces cognitive deficits through augmentation of GABAergic synaptic transmission in the prefrontal cortex},
author = {Monserrat Armenta-Resendiz and Ahlem Assali and Evgeny Tsvetkov and Christopher W Cowan and Antonieta Lavin},
url = {https://pubmed.ncbi.nlm.nih.gov/35788684/},
doi = {10.1038/s41386-022-01371-9},
issn = {1740-634X},
year = {2022},
date = {2022-09-01},
urldate = {2022-09-01},
journal = {Neuropsychopharmacology},
volume = {47},
number = {10},
pages = {1816--1825},
abstract = {Methamphetamine (METH) abuse is associated with the emergence of cognitive deficits and hypofrontality, a pathophysiological marker of many neuropsychiatric disorders that is produced by altered balance of local excitatory and inhibitory synaptic transmission. However, there is a dearth of information regarding the cellular and synaptic mechanisms underlying METH-induced cognitive deficits and associated hypofrontal states. Using PV-Cre transgenic rats that went through a METH sensitization regime or saline (SAL) followed by 7-10 days of home cage abstinence combined with cognitive tests, chemogenetic experiments, and whole-cell patch recordings on the prelimbic prefrontal cortex (PFC), we investigated the cellular and synaptic mechanisms underlying METH-induce hypofrontality. We report here that repeated METH administration in rats produces deficits in working memory and increases in inhibitory synaptic transmission onto pyramidal neurons in the PFC. The increased PFC inhibition is detected by an increase in spontaneous and evoked inhibitory postsynaptic synaptic currents (IPSCs), an increase in GABAergic presynaptic function, and a shift in the excitatory-inhibitory balance onto PFC deep-layer pyramidal neurons. We find that pharmacological blockade of D1 dopamine receptor function reduces the METH-induced augmentation of IPSCs, suggesting a critical role for D1 dopamine signaling in METH-induced hypofrontality. In addition, repeated METH administration increases the intrinsic excitability of parvalbumin-positive fast spiking interneurons (PV + FSIs), a key local interneuron population in PFC that contributes to the control of inhibitory tone. Using a cell type-specific chemogenetic approach, we show that increasing PV + FSIs activity in the PFC is necessary and sufficient to cause deficits in temporal order memory similar to those induced by METH. Conversely, reducing PV + FSIs activity in the PFC of METH-exposed rats rescues METH-induced temporal order memory deficits. Together, our findings reveal that repeated METH exposure increases PFC inhibitory tone through a D1 dopamine signaling-dependent potentiation of inhibitory synaptic transmission, and that reduction of PV + FSIs activity can rescue METH-induced cognitive deficits, suggesting a potential therapeutic approach to treating cognitive symptoms in patients suffering from METH use disorder.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
2021
Wright, Andrew M; Zapata, Agustin; Hoffman, Alexander F; Necarsulmer, Julie C; Coke, Lamarque M; Svarcbahs, Reinis; Richie, Christopher T; Pickel, James; Hope, Bruce T; Harvey, Brandon K; Lupica, Carl R
In: eNeuro, 2021.
@article{WrightENEURO.0017-21.2021,
title = {Effects of Withdrawal from Cocaine Self-Administration on Rat Orbitofrontal Cortex Parvalbumin Neurons Expressing Cre recombinase: Sex-Dependent Changes in Neuronal Function and Unaltered Serotonin Signaling},
author = {Andrew M Wright and Agustin Zapata and Alexander F Hoffman and Julie C Necarsulmer and Lamarque M Coke and Reinis Svarcbahs and Christopher T Richie and James Pickel and Bruce T Hope and Brandon K Harvey and Carl R Lupica},
url = {https://pubmed.ncbi.nlm.nih.gov/34083381/},
doi = {10.1523/ENEURO.0017-21.2021},
year = {2021},
date = {2021-01-01},
urldate = {2021-01-01},
journal = {eNeuro},
publisher = {Society for Neuroscience},
abstract = {The orbitofrontal cortex (OFC) is a brain region involved in higher-order decision-making. Rodent studies show that cocaine self-administration (CSA) reduces OFC contribution to goal-directed behavior and behavioral strategies to avoid drug intake. This change in OFC function persists for many weeks after cocaine withdrawal, suggesting involvement in the process of addiction. The mechanisms underlying impaired OFC function by cocaine are not well-understood. However, studies implicate altered OFC serotonin (5-HT) function in disrupted cognitive processes during addiction and other psychiatric disorders. Thus, it is hypothesized that cocaine impairment of OFC function involves changes in 5-HT signaling, and previous work shows that 5-HT1A and 5-HT2A receptor-mediated effects on OFC pyramidal neurons (PyNs) are impaired weeks after cocaine withdrawal. However, 5-HT effects on other contributors to OFC circuit function have not been fully investigated, including the parvalbumin-containing, fast-spiking interneurons (OFCPV), whose function is essential to normal OFC-mediated behavior. Here, 5-HT function in naive rats and those withdrawn from CSA were evaluated using a novel rat transgenic line in which the rat parvalbumin promoter drives Cre-recombinase expression to permit identification of OFCPV cells by fluorescent reporter protein expression. We find that whereas CSA altered basal synaptic and membrane properties of the OFCPV neurons in a sex-dependent manner, the effects of 5-HT on these cells were unchanged by CSA. These data suggest that the behavioral effects of dysregulated OFC 5-HT function caused by cocaine experience are primarily mediated by changes in 5-HT signaling at PyNs, and not at OFCPV neurons.Significance StatementCocaine addiction involves the inability to change behavior having negative consequences and to adopt beneficial behaviors. The orbitofrontal cortex (OFC) is a brain region involved in this behavioral flexibility, and OFC function is impaired after cocaine use. Moreover, signaling by the neurotransmitter serotonin (5-HT) is impaired in OFC pyramidal neurons (PyNs) after cocaine. However, whether other types of neurons are affected by cocaine is unknown, and we asked whether changes occur in another class of OFC cells known as parvalbumin interneurons. We report that cocaine changed the activity of parvalbumin interneurons in a sex-dependent manner but did not alter 5-HT effects. This suggests that the effects of cocaine on 5-HT function in OFC involves PyNs and not parvalbumin interneurons.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
2020
López-Madrona, Victor J; Pérez-Montoyo, Elena; Álvarez-Salvado, Efrén; Moratal, David; Herreras, Oscar; Pereda, Ernesto; Mirasso, Claudio R; Canals, Santiago
Different theta frameworks coexist in the rat hippocampus and are coordinated during memory-guided and novelty tasks Journal Article
In: eLife, vol. 9, pp. e57313, 2020, ISSN: 2050-084X.
@article{10.7554/eLife.57313,
title = {Different theta frameworks coexist in the rat hippocampus and are coordinated during memory-guided and novelty tasks},
author = {Victor J López-Madrona and Elena Pérez-Montoyo and Efrén Álvarez-Salvado and David Moratal and Oscar Herreras and Ernesto Pereda and Claudio R Mirasso and Santiago Canals},
editor = {Martin Vinck and Laura L Colgin and Antonio Fernandez-Ruiz},
url = {https://pubmed.ncbi.nlm.nih.gov/32687054/},
doi = {10.7554/eLife.57313},
issn = {2050-084X},
year = {2020},
date = {2020-07-01},
journal = {eLife},
volume = {9},
pages = {e57313},
publisher = {eLife Sciences Publications, Ltd},
abstract = {Hippocampal firing is organized in theta sequences controlled by internal memory processes and by external sensory cues, but how these computations are coordinated is not fully understood. Although theta activity is commonly studied as a unique coherent oscillation, it is the result of complex interactions between different rhythm generators. Here, by separating hippocampal theta activity in three different current generators, we found epochs with variable theta frequency and phase coupling, suggesting flexible interactions between theta generators. We found that epochs of highly synchronized theta rhythmicity preferentially occurred during behavioral tasks requiring coordination between internal memory representations and incoming sensory information. In addition, we found that gamma oscillations were associated with specific theta generators and the strength of theta-gamma coupling predicted the synchronization between theta generators. We propose a mechanism for segregating or integrating hippocampal computations based on the flexible coordination of different theta frameworks to accommodate the cognitive needs.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Prasad, Asheeta A; Xie, Caroline; Chaichim, Chanchanok; Nguyen, Jennifer H; McClusky, Hannah E; Killcross, Simon; Power, John M; McNally, Gavan P
Complementary Roles for Ventral Pallidum Cell Types and Their Projections in Relapse Journal Article
In: Journal of Neuroscience, vol. 40, no. 4, pp. 880–893, 2020, ISSN: 0270-6474.
@article{Prasad880,
title = {Complementary Roles for Ventral Pallidum Cell Types and Their Projections in Relapse},
author = {Asheeta A Prasad and Caroline Xie and Chanchanok Chaichim and Jennifer H Nguyen and Hannah E McClusky and Simon Killcross and John M Power and Gavan P McNally},
url = {https://pubmed.ncbi.nlm.nih.gov/31818977/},
doi = {10.1523/JNEUROSCI.0262-19.2019},
issn = {0270-6474},
year = {2020},
date = {2020-01-01},
journal = {Journal of Neuroscience},
volume = {40},
number = {4},
pages = {880--893},
publisher = {Society for Neuroscience},
abstract = {The ventral pallidum (VP) is a key node in the neural circuits controlling relapse to drug seeking. How this role relates to different VP cell types and their projections is poorly understood. Using male rats, we show how different forms of relapse to alcohol-seeking are assembled from VP cell types and their projections to lateral hypothalamus (LH) and ventral tegmental area (VTA). Using RNAScope in situ hybridization to characterize activity of different VP cell types during relapse to alcohol-seeking provoked by renewal (context-induced reinstatement), we found that VP Gad1 and parvalbumin (PV), but not vGlut2, neurons show relapse-associated changes in c-Fos expression. Next, we used retrograde tracing, chemogenetic, and electrophysiological approaches to study the roles of VPGad1 and VPPV neurons in relapse. We show that VPGad1 neurons contribute to contextual control over relapse (renewal), but not to relapse during reacquisition, via projections to LH, where they converge with ventral striatal inputs onto LHGad1 neurons. This convergence of striatopallidal inputs at the level of individual LHGad1 neurons may be critical to balancing propensity for relapse versus abstinence. In contrast, VPPV neurons contribute to relapse during both renewal and reacquisition via projections to VTA. These findings identify a double dissociation in the roles for different VP cell types and their projections in relapse. VPGad1 neurons control relapse during renewal via projections to LH. VPPV neurons control relapse during both renewal and reacquisition via projections to VTA. Targeting these different pathways may provide tailored interventions for different forms of relapse.SIGNIFICANCE STATEMENT Relapse to drug or reward seeking after a period of extinction or abstinence remains a key impediment to successful treatment. The ventral pallidum, located in the ventral basal ganglia, has long been recognized as an obligatory node in a textquoterightfinal common pathwaytextquoteright for relapse. Yet how this role relates to the considerable VP cellular and circuit heterogeneity is not well understood. We studied the cellular and circuit architecture for VP in relapse control. We show that different forms of relapse have complementary VP cellular and circuit architectures, raising the possibility that targeting these different neural architectures may provide tailored interventions for different forms of relapse.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Blog/Comments/Reviews
Last Updated on November 12, 2024
There are 27 surveyed reports for the receiving and usage of the transgenic Pvalb-iCre rats for their research studies.
General Health
There are no reports of general health issues with the LE-Tg(Pvalb-iCre)2Ottc rats
Weight
There are no reports of weight changes with the LE-Tg(Pvalb-iCre)2Ottc rats
Breeding
There are no reports of breeding issues with the LE-Tg(Pvalb-iCre)2Ottc rats
Expression
One researcher observed “leaky” Cre expression in the LE-Tg(Pvalb-iCre)2Ottc rats
Other related rats
Acknowledgements
YaJun Zhang, Julie Necarsulmer, Chris Richie, Brandon Harvey, Janette Lebron