Background | Status & Availability | Transgene Info | Phenotypic Characterization | Breeding | Genotyping | References | Blog/Comments/Reviews | Related rats | Acknowledgements
Background
Glutamate decarboxylase 1 (brain, 67kDa) (GAD67), also known as GAD1, is the enzyme responsible for catalyzing the conversion of L-glutamic acid into gamma-aminobutyric acid (GABA). GABA is the primary inhibitory neurotransmitter in the mammalian brain and GABAergic neurons can be identified by expression of GAD1. Not all GABAergic neurons express GAD1 (some use only GAD2). We have generated and characterized a strain of transgenic Long Evans rats expressing iCre recombinase under a GAD1 promoter (GAD1::iCre, line 3). The tissue-specific expression of iCre can be used in combination with Cre-dependent transgenes to obtain selective expression of transgenes in GAD1(+) neurons.
Status and Availability
This rat has been published (PMID: 28690111).
As of May 17, 2016, this strain is available as line 751 at the RRRC. ![]()
This rat is registered at the Rat Genome Database (RGD) as RGD ID#9588593. ![]()
Transgene Information
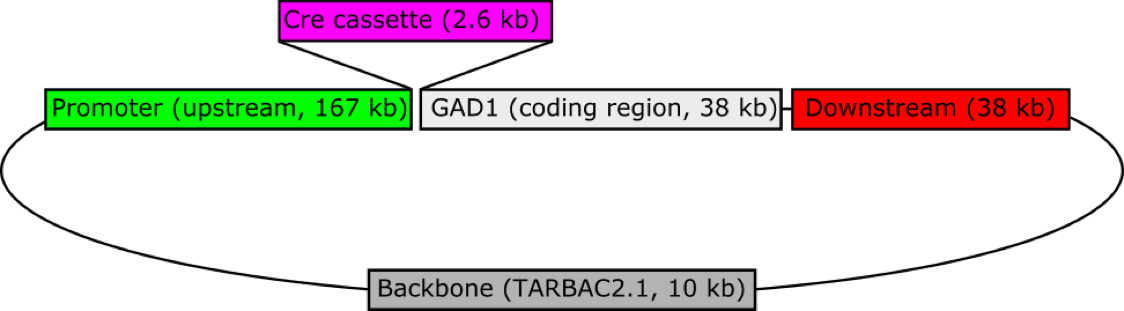
Figure 1: A schematic of the Gad1-iCre transgene. A bacterial artificial chromosome (BAC) containing the rat Gad1 gene (CH230-24D16) was obtained from CHORI, and recombineered to replace the start codon of Gad1 with a 2.6 kb cassette containing iCre (improved Cre recombinase), the polyadenylation signal from the gene for bovine growth hormone, and a galk bacterial selection marker (pOTTC335). This BAC was injected into the pronuclei of fertilized Long Evans rat embryos by NIMH Transgenic Core, and ultimately resulted in 3 independent, phenotypically positive Gad1::iCre lines. This line (LE-Tg(Gad1-iCre)3Ottc) has a single copy of the transgene per haploid genome as determined by droplet digital PCR.
Phenotypic Characterization
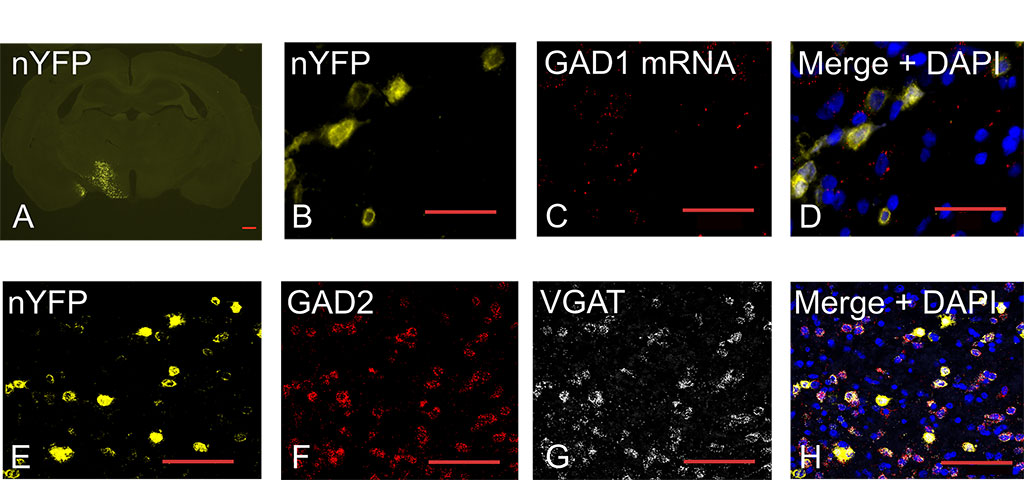
Figure 2: Basic Characterization of the GAD-Cre Rat. (A) Coronal section showing nuclear YFP (nYFP) expression in LH 2 weeks after AAV injection. The scale bar represents 0.5 mm. Colocalization of (B) nYFP protein fluorescence (C) and GAD1 mRNA. (D) Merged image of nYFP (yellow), GAD1 mRNA (red), and total nuclei (blue; DAPI) shows GAD1 mRNA associates with nYFP signal in the LH. (E–G) Colocalization of (E) nYFP protein fluorescence, (F) GAD2 mRNA, and (G) VGAT mRNA. (H) Merged image of nYFP (yellow), GAD2, and VGAT; scale bars (C–I) = 100 μm. Download print resolution version here.
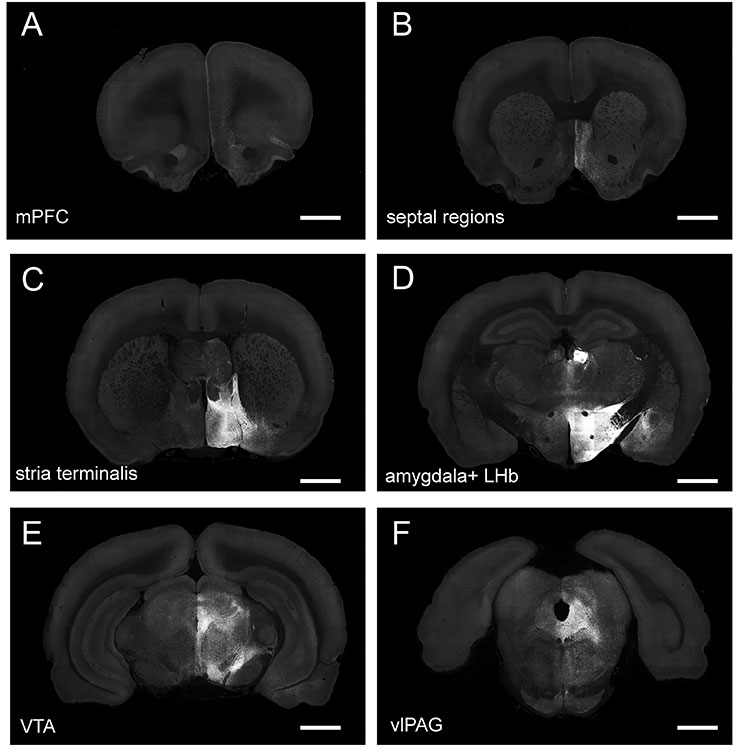
Figure 3: Projections from virally transduced GABAergic neurons in lateral hypothalamus. AAV1-EF1a-DIO-mem-AcGFP was injected into the LH, and brain tissue was imaged 2 weeks later using whole-brain TissueCyte system. There was intense membrane-GFP labeling in LH and lateral habenula and amygdala (D) and in the bed nucleus of the stria terminalis (C). Membrane-GFP labeling was also observed in VTA within the parabrachial pigmented area (E), septal regions (B), ventral-lateral periaqueductal gray (F), and lateral infralimbic (IL) and prelimbic (PL) cortices (mPFC; A). Views of stitched fields of coronal sections are shown. The scale bar represents 2 mm. Download print resolution version here.
Figures were modified and reprinted from “Sharpe et. al, Lateral Hypothalamic GABAergic Neurons Encode Reward Predictions that Are Relayed to the Ventral Tegmental Area to Regulate Learning. Curr Biol. 2017 Jul 24;27(14):2089-2100.e5. doi: 10.1016/j.cub.2017.06.024. Epub 2017 Jul 6” with permission from Elsevier.
For more details and data on phenotypic characterization, see reference (PMID: 28690111).
Breeding Strategy
Breeding Information, click here for PDF
Genotyping Assays
Assay for the presence of Gad1-iCre transgene, click here for PDF
References that cite this rat
2022
Ventral pallidum GABA neurons bidirectionally control opioid relapse across rat behavioral models Journal Article
In: Addict Neurosci, vol. 3, 2022, ISSN: 2772-3925.
2020
An approach for long-term, multi-probe Neuropixels recordings in unrestrained rats Journal Article
In: eLife, vol. 9, pp. e59716, 2020, ISSN: 2050-084X.
Complementary Roles for Ventral Pallidum Cell Types and Their Projections in Relapse Journal Article
In: Journal of Neuroscience, vol. 40, no. 4, pp. 880–893, 2020, ISSN: 0270-6474.
2018
Distinct Accumbens Shell Output Pathways Promote versus Prevent Relapse to Alcohol Seeking Journal Article
In: Neuron, vol. 98, no. 3, pp. 512–520.e6, 2018, ISBN: 0896-6273.
2017
Lateral Hypothalamic GABAergic Neurons Encode Reward Predictions that Are Relayed to the Ventral Tegmental Area to Regulate Learning. Journal Article
In: Curr Biol, vol. 27, no. 14, pp. 2089–2100, 2017, ISSN: 1879-0445 (Electronic); 0960-9822 (Linking), (*First paper describing LE-Tg(GAD1-iCre)3Ottc rat.).
Blog/Comments/Reviews
Last Updated on November 12, 2024
There are 26 surveyed reports for the receiving and usage of the transgenic GAD1-iCre rats with only 3 total reported issues.
General Health
One lab group noticed patches of fur loss at 4 months of age in rats that was resolved in about 2 weeks.
Another lab group discovered a large tumor in one of the original GAD1-iCre breeders.
Weight
There are no reports of weight issues with the LE-Tg(GAD-iCre)3Ottc rats
Breeding
There are no reports of breeding issues with the LE-Tg(GAD1-iCre)3Ottc rats
Expression
One lab group experienced variable Cre expression with inconsistencies in Cre and GAD1 labeled positive neurons when examining the nucleus accumbens core, ventral pallidum, and dorsal raphe brain regions
*Please be sure to follow the breeding guide to produce a working LE-Tg(Drd1a-iCre)3Ottc colony.
Other related rats
Acknowledgements
YaJun Zhang, Julie Necarsulmer, Chris Richie, Brandon Harvey, Janette Lebron, Melissa Sharpe, Geoffrey Schoenbaum