Background | Status & Availability | Transgene Info | Phenotypic Characterization | Breeding | Genotyping | References | Blog/Comments/Reviews | Related rats | Acknowledgements
Background
The dopamine receptor Drd2 is highly expressed in medium spiny neurons (MSNs) of the striatum. The Drd2-expressing MSNs send inhibitory projections to the globus pallidus externa (GPe) which in turn sends inhibitory projections to the sub-thalamic nucleus (STN). The STN neurons have excitory projections that activate basal ganglia structures such as the globus pallidus interna (GPi) and substantia nigra reticulata (SNr). This collection of projecting neurons is referred to as the “indirect pathway” or “D2 pathway”. The indirect pathway is important for controlling voluntary movements and reward-related behaviors. We have generated and characterized a strain of transgenic Long Evans rats expressing iCre recombinase under a Drd2 promoter referred to as “LE-Tg(Drd2-iCre)1Ottc.” The tissue-specific expression of iCre can be used in combination with Cre-dependent transgenes to obtain selective expression of transgenes in Drd2(+) cells.
Status and Availability
This strain has been published (PMID: 33051346).
As of February 21, 2017, this strain is available as line 768 at the RRRC.
This rat is registered at the Rat Genome Database (RGD) as RGD ID#10412327.
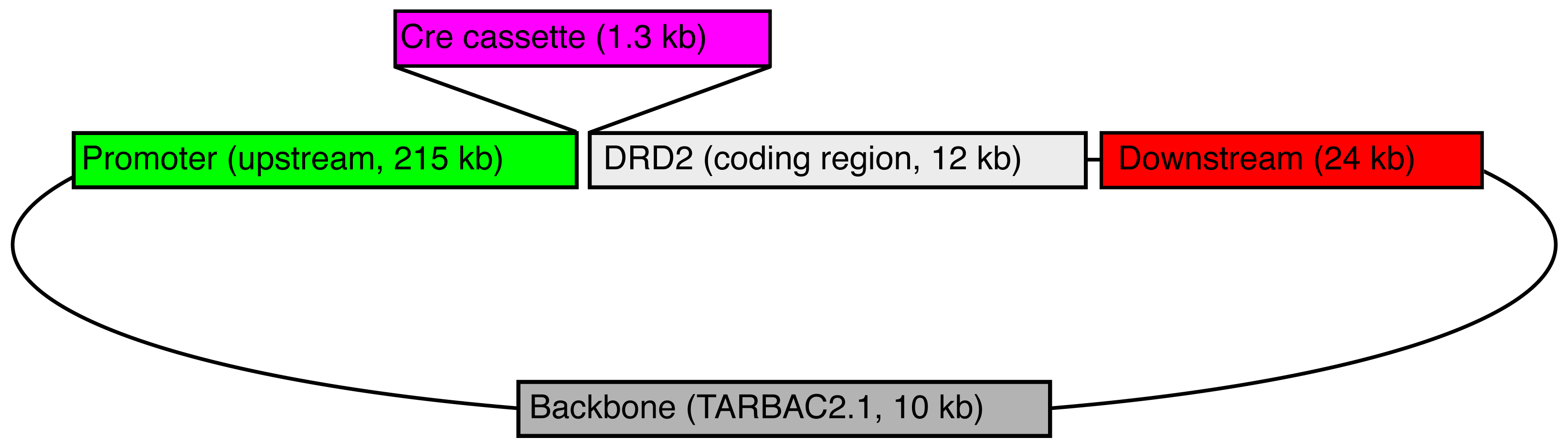
Transgene Information
Legend: A bacterial artificial chromosome (BAC) containing the rat Drd2 gene (CH230-11B15) was obtained from CHORI, and recombineered to replace the start codon of Drd2 with a 1.3 kb cassette containing iCre (improved Cre recombinase), the polyadenylation signal from the gene for bovine growth hormone, and a galk bacterial selection marker (pOTTC464). This BAC was injected into the pronuclei of fertilized Long Evans rat embryos by NIMH Transgenic Core, and ultimately resulted in two independent, phenotypically positive Drd2-iCre lines. This line (LE-Tg(Drd2-iCre)1Ottc) has four copies of the transgene per haploid genome as determined by droplet digital PCR.
Phenotypic Characterization
To be added. Please check back.
Breeding Strategy
Breeding Information, click here for PDF
Genotyping Assays
Assay for the presence of Drd2 and iCre, click here for PDF
References that cite this rat
2023
Circuit and Cell-Specific Contributions to Decision Making Involving Risk of Explicit Punishment in Male and Female Rats Journal Article
In: J Neurosci, vol. 43, no. 26, pp. 4837–4855, 2023, ISSN: 1529-2401.
In: Neuropsychopharmacology, vol. 48, no. 3, pp. 459–467, 2023, ISSN: 1740-634X.
2020
Chemogenetic selective manipulation of nucleus accumbens medium spiny neurons bidirectionally controls alcohol intake in male and female rats Journal Article
In: Scientific Reports, vol. 10, no. 1, pp. 19178, 2020, ISBN: 2045-2322.
An approach for long-term, multi-probe Neuropixels recordings in unrestrained rats Journal Article
In: eLife, vol. 9, pp. e59716, 2020, ISSN: 2050-084X.
In: Journal of Neuroscience, vol. 40, no. 44, pp. 8463–8477, 2020, ISSN: 0270-6474.
Blog/Comments/Reviews
Last Updated on November 12, 2024
There are 36 surveyed reports for the receiving and usage of the transgenic Drd2a-iCre rats to conduct scientific experiments.
General Health
One report found that the Drd2-iCre homozygotes perished early in terms of a laboratory rat’s lifespan.
Weight
Less than 10% of reports observed weight differences between the male transgenic and wild type rats
- LE male rats were ~20% greater than the transgenic Drd2-iCre rats
- LE female rats were ~28% greater than the transgenic Drd2-iCre rats
- Another laboratory reported Drd2-iCre rats were overweight in comparison to WT and Drd1 rats
Breeding
Less than 10% of reports observed smaller litter sizes with 1 lab specifically noticed that the transgenic female with male wild type produced a smaller litter.
Expression
Less than 10% of reports experienced either minimal or unusual Cre expression when completing their detection-based assays.
*Please be sure to follow the breeding guide to produce a working LE-Tg(Drd2a-iCre)3Ottc colony.
Other related rats
Acknowledgements
YaJun Zhang, Julie Necarsulmer, Chris Richie, Brandon Harvey, Janette Lebron