Background | Status & Availability | Transgene Info | Phenotypic Characterization | Breeding | Genotyping | References | Blog/Comments/Reviews | Related rats | Acknowledgements
Background
The LE-Tg(cFos-eGFP)2Ottc rat is a reporter rat expressing enhanced green fluorescent protein (eGFP) in cells that are activated to express the immediate early gene Fos. This rat was made by injecting a previously described DNA construct (Barth et al J Neurosci 2004; map shown below) into fertilized eggs from Long Evans rats. Founders with incorporated transgene were screened for eGFP expression. This line can be compared to “c-Fos-GFP” transgenic rat (described by Cifani et al. 2012, J Neurosci ![]() ) as it is an independent founder from the injection mix, however LE-Tg(cFos-eGFP)2Ottc rats show more expression in caudal brain areas compared to the “c-Fos-GFP” rat described by Cifani et al.
) as it is an independent founder from the injection mix, however LE-Tg(cFos-eGFP)2Ottc rats show more expression in caudal brain areas compared to the “c-Fos-GFP” rat described by Cifani et al.
Status and Availability
This rat has been published (PMID: 30521931).
As of March 1, 2017, this strain is available as line #00766 at the RRRC. ![]()
This rat is registered at the Rat Genome Database (RGD) as RGD ID# 9588570. ![]()
Transgene Information
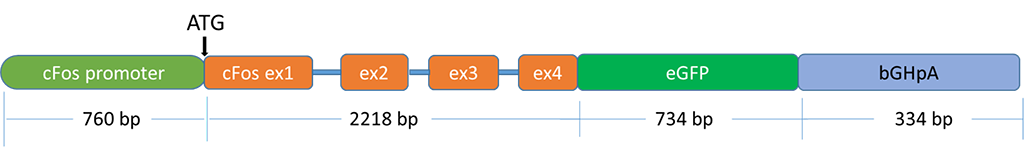
Figure 1. Schematic of the cFos-eGFP transgene. The mouse cFos promoter as well as the first 4 exons of the cFos transcript were translationally-fused to enhanced green fluorescent protein (eGFP) followed by a bovine growth hormone polyadenylation signal. This fragment was liberated from the plasmid “c-fos-gfp” (a gift from Dr. Alison Barth, Carnegie Mellon University, Pittsburgh, PA  ) using HindIII and KpnI restriction enzymes, and then injected into pronuclei of fertilized Long Evans rat embryos by NIMH Transgenic Core. Two founders were identified that carry the transgene but only one showed a eGFP-expressing phenotype. The copy number and integration(s) have not been characterized for LE-Tg(cFos-eGFP)2Ottc.
) using HindIII and KpnI restriction enzymes, and then injected into pronuclei of fertilized Long Evans rat embryos by NIMH Transgenic Core. Two founders were identified that carry the transgene but only one showed a eGFP-expressing phenotype. The copy number and integration(s) have not been characterized for LE-Tg(cFos-eGFP)2Ottc.
Phenotypic Characterization
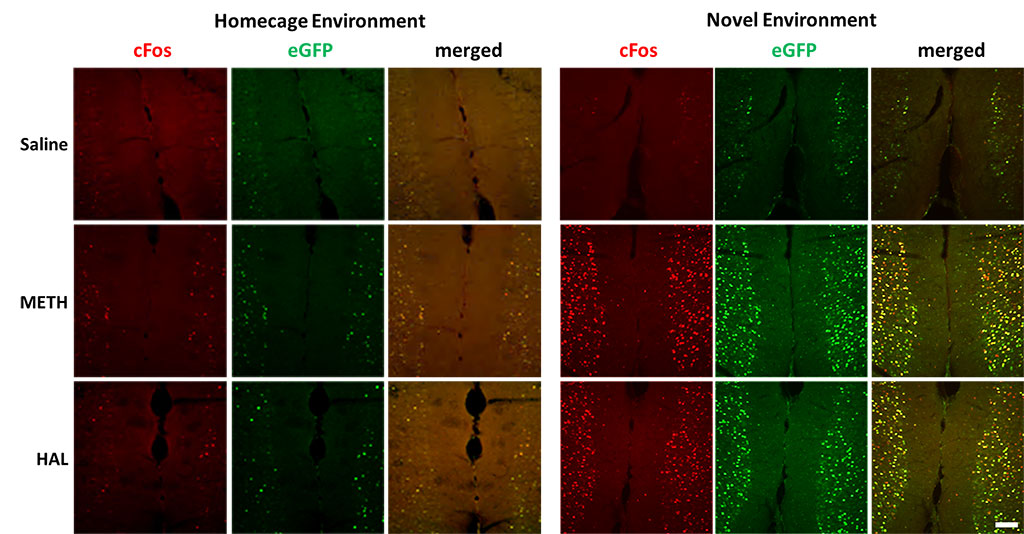
Figure 2. Activation of endogenous cFos expression and eGFP expression in prefrontal cortex. Male LE-Tg(cFos-eGFP)2Ottc rats were injected with saline, methamphetamine (METH; 2.5 mg/kg) or haloperidol (HAL; 1 mg/kg) and immediately placed into home cage or novel environment. Two hours later, animals were perfused with 4% PFA and cryosectioned (40 μm). Sections were immunostained for cFos overnight at 4C. Two hours of secondary antibody. Immunolabeled sections were mounted on slides and imaged for eGFP fluorescence and cFos immunoreactivity using NIKON upright microscope equipped with epifluorescence. Scale bar – 100 μm. Download print resolution version here.
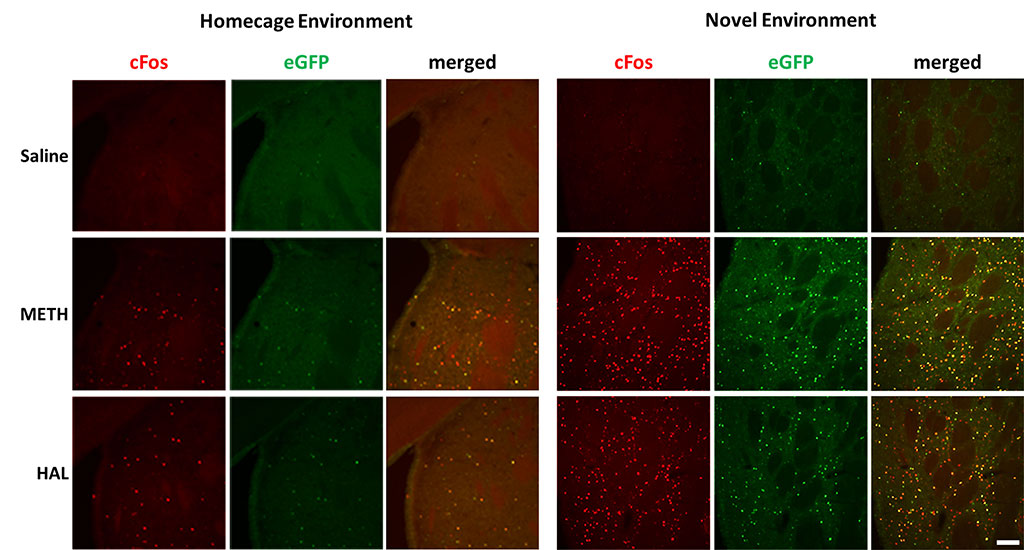
Figure 3. Activation of endogenous cFos expression and eGFP expression in dorsal striatum. Male LE-Tg(cFos-eGFP)2Ottc rats were injected with saline, methamphetamine (METH; 2.5 mg/kg) or haloperidol (HAL; 1 mg/kg) and immediately placed into home cage or novel environment. Two hours later, animals were perfused with 4% PFA and cryosectioned (40 μm). Sections were immunostained for cFos overnight at 4C. Two hours of secondary antibody. Immunolabeled sections were mounted on slides and imaged for eGFP fluorescence and cFos immunoreactivity using NIKON upright microscope equipped with epifluorescence. Scale bar – 100 μm. Download print resolution version here.
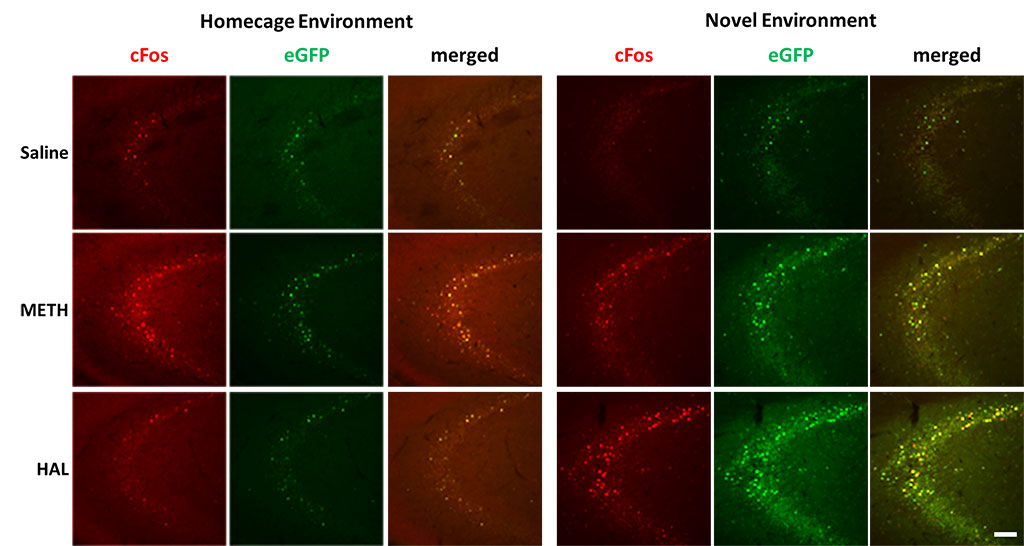
Figure 4. Activation of endogenous cFos expression and eGFP expression in hippocampus. Male LE-Tg(cFos-eGFP)2Ottc rats were injected with saline, methamphetamine (METH; 2.5 mg/kg) or haloperidol (HAL; 1 mg/kg) and immediately placed into home cage or novel environment. Two hours later, animals were perfused with 4% PFA and cryosectioned (40 μm). Sections were immunostained for cFos overnight at 4C. Two hours of secondary antibody. Immunolabeled sections were mounted on slides and imaged for eGFP fluorescence and cFos immunoreactivity using NIKON upright microscope equipped with epifluorescence. Scale bar – 100 μm. Download print resolution version here.
Experiments conducted at the NIDA IRP by Bruce Hope, YaJun Zhang and Brandon Harvey.
Additional phenotypic characterization is recommended for brain region and experimental paradigm of interest.
Breeding Strategy
Breeding Information, click here for PDF
Genotyping Assays
Protocol for genotyping transgenic rats, click here for PDF
References that cite this rat
2019
In: Behavioural Brain Research, vol. 360, pp. 134 - 145, 2019, ISSN: 0166-4328.
Blog/Comments/Reviews
Last Updated on November 12, 2024
LE-Tg(cFos-eGFP)2Ottc
There are 4 surveyed reports for the receiving and usage of the Long Evans Transgenic cFos-eGFP rats reported an issue during the breeding process.
General Health
There are no reports of general health issues with the LE-Tg(cFos-eGFP)2Ottc rats
Weight
There are no reports of general health issues with the LE-Tg(cFos-eGFP)2Ottc rats
Breeding
One laboratory noticed that the breeding took longer than usual, and they did not receive their first litter until 2-3 months after breeding the LE transgenic male and LE wildtype female rat. This issue only occurred in the F0 within their breeding colony, and it is speculated that the long-distance transport of the transgenics (> 5000 km) may have influenced the breeding delay.
Expression
There are no reports of expression issues with the LE-Tg(cFos-eGFP)2Ottc rats
Other related rats
LE-Tg(cFos-eGFP)1Ottc (aka “c-Fos-GFP” rat)
Published as the “c-Fos-GFP” rat which is also a randomly integrated transgene on a Long Evans background that is described by Cifani et al 2012 J Neurosci. If you are interested in the “c-Fos-GFP” rat, please contact Bruce Hope (bhope@intra.nida.nih.gov).
Acknowledgements
YaJun Zhang, Julie Necarsulmer, Christopher T. Richie, Brandon Harvey, Janette Lebron