Hot Off the Press – April 24, 2024
Published in Molecular Psychiatry by Atul Daiwile and Jean Lud Cadet, et al. from the NIDA IRP Molecular Neuropsychiatry Section.
Summary
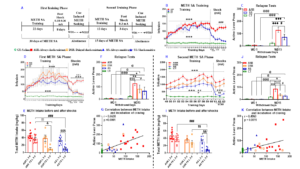
Methamphetamine also called METH, crank, ice etc. is a powerful stimulant that has caused addiction in a lot of people in the world. Humans who take too much methamphetamine develop many negative problems on the brain. Some people develop major psychiatric problems, but there is no approved treatment for the problems caused by methamphetamine. This might be because how methamphetamine works in the brain to cause addiction. Our laboratory has been studying how methamphetamine can cause changes in the way the brain functions in rats with a history of methamphetamine use. In this paper, rats that have experience giving themselves methamphetamine were given the opportunity to earn or to stop taking the drug when they were exposed to footshocks through an electrical grid on the floor of a cage where they take methamphetamine. We also tried to figure out if some rats would continue or stop taking the drug after they had not had access for a period of time. We did this because some users will stop taking the drug by themselves or because they are forced to stop because they are hospitalized or arrested by the police. This is the first time that any group of scientists has found that animals that kept taking methamphetamine in the face of punishment with footshocks will continue to take methamphetamine even if their access to the drug was blocked for a period of time. We also found that some rats that had decreased their methamphetamine taking behaviors in the presence of punishment will now take the drug even when they face adverse consequences after a period of not having access to drug. Importantly, we also found that the 3 groups of rats have different changes in the expression of several genes in a brain area called dorsal striatum which is involved in some aspects of addiction. One of these genes is called brain-derived neurotrophic factor (BDNF) which is influenced by an epigenetic enzyme called histone deacetylase-2 (HDAC2). This animal model of methamphetamine use disorder (addiction) has the potential to help us understand the way animals, and by extension, humans, take drug non-stop even when they are faced with bad medical or legal consequences.
Publication Information
In: Mol Psychiatry, 2024, ISSN: 1476-5578.