Hot Off the Press – July 8, 2022
Published in Pharmacological Research by Paulo De Oliveira, Ph.D. and Sergi Ferré, M.D., Ph.D. of the NIDA-IRP Integrative Neurobiology Section.
Summary
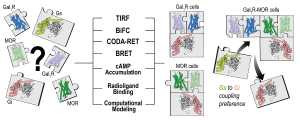
We recently demonstrated that complexes (heteromers) of μ-opioid receptors (MORs) and receptors for the neuropeptide galanin of the Gal1subtype (Gal1Rs) localized in the brainstem (in the ventral tegmental area) constitute a main population of MORs that mediate the dopaminergic effects of opioids (dopamine release and euphoric effects). In the present study, using several in vitro and in silico biophysical, biochemical and computational techniques, we determine the preferred quaternary structure of MORs and Gal1Rs when expressed alone and when forming heteromers. When expressed alone, MORs and Gal1Rs are dimers coupled to one inhibitory Gi protein and to one stimulatory Gs protein, respectively. When co-expressed, they form heterotetramers with a MOR dimer coupled to Gi and, unexpectedly, a Gal1R dimer coupled to Gs. The study describes, for the first time, the mechanism of this heteromerization-dependent switch in G protein coupling, which is related to a change in the dimeric interface of the Gal1R in the MOR-Gal1R heteromer, and which converts galanin into an excitatory neurotransmitter that promotes cAMP accumulation.
Publication Information
Preferential Gs protein coupling of the galanin Gal1 receptor in the µ-opioid-Gal1 receptor heterotetramer Journal Article
In: Pharmacol Res, vol. 182, pp. 106322, 2022, ISSN: 1096-1186.