 Hot Off the Press – October 15, 2024
Hot Off the Press – October 15, 2024
Published in Nature Communications with contributions from Alessandro Bonifazi , Francisco Battiti and Amy Hauck Newman of the NIDA IRP Medicinal Chemistry Section.
Summary
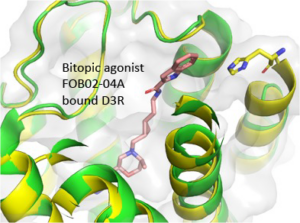
We have been designing bitopic ligands for more than two decades with the idea that the secondary pharmacophore would bind in a site that is exclusive and thus render our compounds highly selective for our target. Although we and others have been successful at using the bitopic strategy for antagonists and partial agonists, it has been much more challenging to discover highly dopamine D3 receptor (D3R)-selective agonists. In this paper, a cryoEM structure reveals that our D3R-selective agonist, FOB02-04A, accesses a secondary binding pocket of D3R with its extended secondary pharmacophore, which renders this compound D3R-selective. Moreover, a Histamine (H29) residue in Transmembrane (TM) 1 was identified that is only available to agonists that can reach it. This residue is far from the orthosteric binding site where nonselective agonists, including dopamine, bind. When FOB02-04A interacts with this H29, it is not only selective over the highly homologous dopamine D2 receptor (D2R), but is fully efficacious, as this residue dictates efficacy, another finding from this structure that was thus far unknown. Indeed, replacing the H29 with multiple other amino acid residues renders the D3R inactive. These findings have not only identified critical residues for selectivity and efficacy but open the opportunity for new drug design across Class A G-protein coupled receptors, never before revealed.
Publication Information
A bitopic agonist bound to the dopamine 3 receptor reveals a selectivity site Journal Article
In: Nat Commun, vol. 15, no. 1, pp. 7759, 2024, ISSN: 2041-1723.
