 Hot Off the Press – August 15, 2022
Hot Off the Press – August 15, 2022
Summary
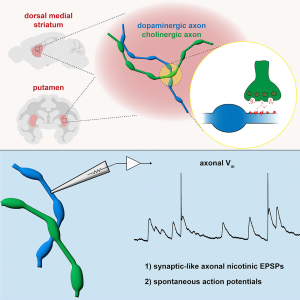
Communication between brain cells (neurons) generally involves the unidirectional flow of information. Input from upstream cells arrives onto the dendrites, gets processed in the cell body where it is converted into a rapid electrical wave (action potential) that travels down the axon to trigger the release of neurotransmitter molecules from axon terminals onto the dendrites of downstream cells. The signal is passed from one cell to the next at the synapse, which is a structure where the axon of one cell and dendrite of another nearly touch. However, dopamine-releasing neurons seem to take a shortcut: the axons of dopaminergic neurons receive input directly from upstream acetylcholine-releasing neurons that may influence the neurotransmitter release of dopaminergic terminals. Until now, how this communication occurs between cells that bypasses dendrites was a mystery. In a recent trans-NIH collaborative study led by Dr. Zayd Khaliq (NINDS, jointly appointed at NIDA), the research team made technically challenging recordings of electrical signals from the midbrain dopaminergic axons. Although dopaminergic axons lack classic synapses, signaling onto axons of dopaminergic neurons occurs very much like what happens in a traditional dendritic synapse, including the ability to initiate spontaneous axonal action potentials. This observation goes against the classical notion of unidirectional flow of information in the nervous system. This phenomenon was observed in rodents and also in Rhesus macaque brains and provides a new framework for understanding the flow of information in the brain pointing to possible new targets for treating substance use disorders and Parkinson’s Disease.
Publication Information
Synaptic-like axo-axonal transmission from striatal cholinergic interneurons onto dopaminergic fibers Journal Article
In: Neuron, 2022, ISSN: 1097-4199.
