Featured Paper of the Month – September 2018.
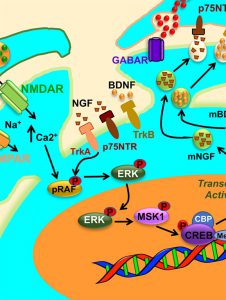 Methamphetamine addiction is a public health threat throughout the world. Investigators in Dr. Cadet’s laboratory in the intramural program have developed a rat model of methamphetamine addiction that includes one of the psychiatric criteria used to make that diagnosis in humans. In that model, they used footshocks to represent adverse consequences that are present during the clinical course of addiction. Contingent footshocks helped the investigators to identify some rats (addicted) that continue to press a lever to get methamphetamine whereas other rats (non-addicted) suppress or stop their taking of methamphetamine in the presence of these adverse consequences. The investigators wanted to know if there were chemicals that could help them understand why these animals behaved differently in the presence of these shocks. They focused their attention on some chemicals called trophic factors including brain derived neurotrophic factor (BDNF) that are thought to regulate drug taking by rats. Because one area of the brain called dorsal striatum is involved in habit forming similar to the habits observed in patients who abuse drugs, the researchers measured the expression of some of these factors in that brain structure. They found that BDNF protein levels were similarly increased in the striatum in both groups of rats. However, another trophic factor called nerve growth factor (NGF) showed greater increases in the non-addicted rats. They also found that the receptor for NGF, called TrkA, is only activated in the non-addicted animals. Another important receptor for NGF called p75NTR is also only activated in the non-addicted rats. Importantly, proteins that are usually activated in cells that are stimulated by NGF were also activated only in the striatum of the non-addicted animals. These observations indicate that stimulation of NGF and other NGF-dependent proteins play a role in the suppression of methamphetamine taking by the non-addicted rats. These data also suggest that there is a need to develop medications that can activate NGF-dependent mechanisms that might benefit patients who are addicted to methamphetamine.
Methamphetamine addiction is a public health threat throughout the world. Investigators in Dr. Cadet’s laboratory in the intramural program have developed a rat model of methamphetamine addiction that includes one of the psychiatric criteria used to make that diagnosis in humans. In that model, they used footshocks to represent adverse consequences that are present during the clinical course of addiction. Contingent footshocks helped the investigators to identify some rats (addicted) that continue to press a lever to get methamphetamine whereas other rats (non-addicted) suppress or stop their taking of methamphetamine in the presence of these adverse consequences. The investigators wanted to know if there were chemicals that could help them understand why these animals behaved differently in the presence of these shocks. They focused their attention on some chemicals called trophic factors including brain derived neurotrophic factor (BDNF) that are thought to regulate drug taking by rats. Because one area of the brain called dorsal striatum is involved in habit forming similar to the habits observed in patients who abuse drugs, the researchers measured the expression of some of these factors in that brain structure. They found that BDNF protein levels were similarly increased in the striatum in both groups of rats. However, another trophic factor called nerve growth factor (NGF) showed greater increases in the non-addicted rats. They also found that the receptor for NGF, called TrkA, is only activated in the non-addicted animals. Another important receptor for NGF called p75NTR is also only activated in the non-addicted rats. Importantly, proteins that are usually activated in cells that are stimulated by NGF were also activated only in the striatum of the non-addicted animals. These observations indicate that stimulation of NGF and other NGF-dependent proteins play a role in the suppression of methamphetamine taking by the non-addicted rats. These data also suggest that there is a need to develop medications that can activate NGF-dependent mechanisms that might benefit patients who are addicted to methamphetamine.
Publication Information
In: Int J Neuropsychopharmacol, vol. 21, no. 3, pp. 281–290, 2018, ISSN: 1469-5111 (Electronic); 1461-1457 (Linking).
