Featured Paper of the Month – September 2017
Geng, Xiujuan; Hu, Yuzheng; Gu, Hong; Salmeron, Betty Jo; Adinoff, Bryon; Stein, Elliot A; Yang, Yihong
Salience and default mode network dysregulation in chronic cocaine users predict treatment outcome. Journal Article
In: Brain, vol. 140, no. 5, pp. 1513–1524, 2017, ISSN: 1460-2156 (Electronic); 0006-8950 (Linking).
@article{Geng2017,
title = {Salience and default mode network dysregulation in chronic cocaine users predict treatment outcome.},
author = {Xiujuan Geng and Yuzheng Hu and Hong Gu and Betty Jo Salmeron and Bryon Adinoff and Elliot A Stein and Yihong Yang},
url = {https://www.ncbi.nlm.nih.gov/pubmed/28334915},
doi = {10.1093/brain/awx036},
issn = {1460-2156 (Electronic); 0006-8950 (Linking)},
year = {2017},
date = {2017-05-01},
journal = {Brain},
volume = {140},
number = {5},
pages = {1513--1524},
address = {Neuroimaging Research Branch, National Institute on Drug Abuse, Intramural Research Program, National Institutes of Health, Baltimore, MD, USA.},
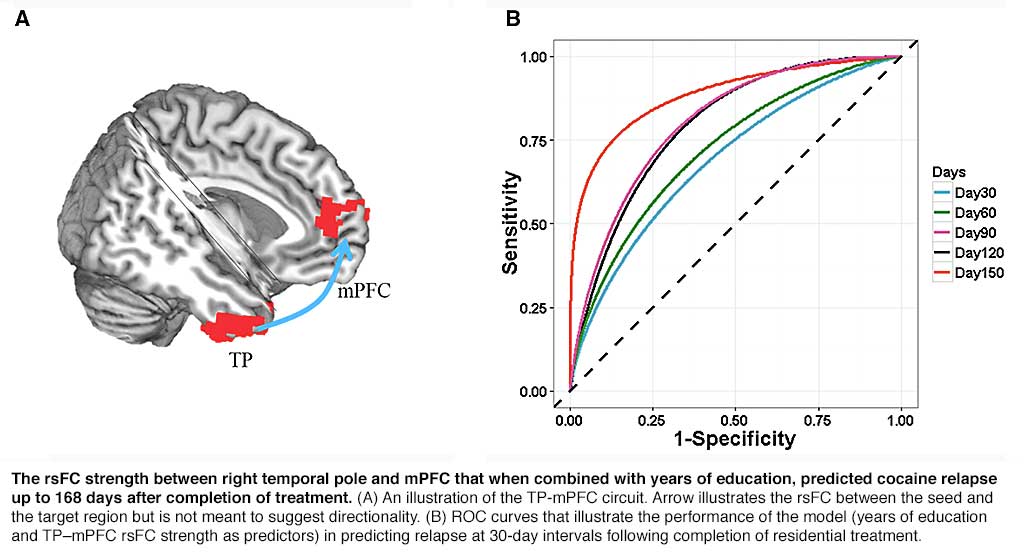
abstract = {While chronic cocaine use is associated with abnormalities in both brain structure and function within and interactions between regions, previous studies have been limited to interrogating structure and function independently, and the detected neural differences have not been applied to independent samples to assess the clinical relevance of results. We investigated consequences of structural differences on resting-state functional connectivity in cocaine addiction and tested whether resting-state functional connectivity of the identified circuits predict relapse in an independent cohort. Subjects included 64 non-treatment-seeking cocaine users (NTSCUs) and 67 healthy control subjects and an independent treatment-completed cohort (n = 45) of cocaine-dependent individuals scanned at the end of a 30-day residential treatment programme. Differences in cortical thickness and related resting-state functional connectivity between NTSCUs and healthy control subjects were identified. Survival analysis, applying cortical thickness of the identified regions, resting-state functional connectivity of the identified circuits and clinical characteristics to the treatment cohort, was used to predict relapse. Lower cortical thickness in bilateral insula and higher thickness in bilateral temporal pole were found in NTSCUs versus healthy control subjects. Whole brain resting-state functional connectivity analyses with these four different anatomical regions as seeds revealed eight weaker circuits including within the salience network (insula seeds) and between temporal pole and elements of the default mode network in NTSCUs. Applying these circuits and clinical characteristics to the independent cocaine-dependent treatment cohort, functional connectivity between right temporal pole and medial prefrontal cortex, combined with years of education, predicted relapse status at 150 days with 88% accuracy. Deficits in the salience network suggest an impaired ability to process physiologically salient events, while abnormalities in a temporal pole-medial prefrontal cortex circuit might speak to the social-emotional functional alterations in cocaine addiction. The involvement of the temporal pole-medial prefrontal cortex circuit in a model highly predictive of relapse highlights the importance of social-emotional functions in cocaine dependence, and provides a potential underlying neural target for therapeutic interventions, and for identifying those at high risk of relapse.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
While chronic cocaine use is associated with abnormalities in both brain structure and function within and interactions between regions, previous studies have been limited to interrogating structure and function independently, and the detected neural differences have not been applied to independent samples to assess the clinical relevance of results. We investigated consequences of structural differences on resting-state functional connectivity in cocaine addiction and tested whether resting-state functional connectivity of the identified circuits predict relapse in an independent cohort. Subjects included 64 non-treatment-seeking cocaine users (NTSCUs) and 67 healthy control subjects and an independent treatment-completed cohort (n = 45) of cocaine-dependent individuals scanned at the end of a 30-day residential treatment programme. Differences in cortical thickness and related resting-state functional connectivity between NTSCUs and healthy control subjects were identified. Survival analysis, applying cortical thickness of the identified regions, resting-state functional connectivity of the identified circuits and clinical characteristics to the treatment cohort, was used to predict relapse. Lower cortical thickness in bilateral insula and higher thickness in bilateral temporal pole were found in NTSCUs versus healthy control subjects. Whole brain resting-state functional connectivity analyses with these four different anatomical regions as seeds revealed eight weaker circuits including within the salience network (insula seeds) and between temporal pole and elements of the default mode network in NTSCUs. Applying these circuits and clinical characteristics to the independent cocaine-dependent treatment cohort, functional connectivity between right temporal pole and medial prefrontal cortex, combined with years of education, predicted relapse status at 150 days with 88% accuracy. Deficits in the salience network suggest an impaired ability to process physiologically salient events, while abnormalities in a temporal pole-medial prefrontal cortex circuit might speak to the social-emotional functional alterations in cocaine addiction. The involvement of the temporal pole-medial prefrontal cortex circuit in a model highly predictive of relapse highlights the importance of social-emotional functions in cocaine dependence, and provides a potential underlying neural target for therapeutic interventions, and for identifying those at high risk of relapse.