 Featured Paper of the Month – October 2019.
Featured Paper of the Month – October 2019.
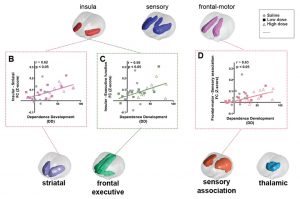
Smoking remains a major public health burden, with approximately 20% of the world’s population engaging in regular smoking. Given the high relapse rate among smokers who enter treatments programs, early identification of vulnerable individuals, before the conversion from casual experimentation to regular smoking and addiction, is an important milestone to understand, prevent and potentially minimize nicotine dependence. Using a rodent model of nicotine dependence, we developed a quantitative predictor of subsequent nicotine dependence severity using graph theory-based analyses of fMRI BOLD resting state data collected at baseline, prior to any drug experience. Using an entirely data driven, hypothesis-free analytic strategy, we observed that connectivity between insular regions before drug exposure can predict dependence severity following subsequent nicotine exposure. This finding is in stark contrast to current biomarkers that have observed correlative relationships between brain connectivity and addiction-related behaviors observed after the onset and progression of a substance use disorder. The results of our preclinical model offer a novel finding of a predictive biomarker of addiction severity and provide a framework upon which intervention-based and mechanistic experiments can investigate the role of these brain modules in nicotine dependence, relapse and successful abstinence.
Publication Information
Intrinsic Insular-Frontal Networks Predict Future Nicotine Dependence Severity. Journal Article
In: J Neurosci, vol. 39, no. 25, pp. 5028–5037, 2019, ISSN: 1529-2401 (Electronic); 0270-6474 (Linking).
