 Featured Paper of the Month – May 2023
Featured Paper of the Month – May 2023
Published in PNAS with contributions from Carl Lupica, Alexander Hoffman, and Michael Baumann of the NIDA IRP.
Summary
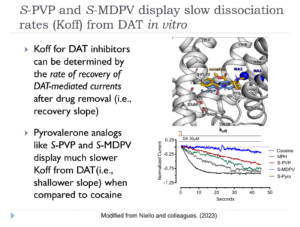
Pyrovalerone analogs, like αPVP (i.e., “flakka”) and MDPV (i.e., “bath salts”), are abused stimulant drugs that produce serious and sustained adverse effects in humans. It is well established that αPVP and MDPV bind to the dopamine transporter (DAT), but the precise time-course of drug binding has not been studied. Here we demonstrate that various DAT inhibitors can be distinguished based on their in vitro binding kinetics, whereby the dissociation rate (i.e., Koff) of a drug from DAT in vitro predicts the duration of its stimulant effects in vivo. In particular, we show that S-isomers of αPVP and MDPV display slow dissociation from DAT and sustained psychostimulant effects in mice, which differs from the fast in vitro kinetics and short-lived effects of drugs like cocaine. Our study provides key evidence that binding kinetics at DAT are an important, yet often overlooked, determinant of psychostimulant drug action.
Publication Information
Persistent binding at dopamine transporters determines sustained psychostimulant effects Journal Article
In: Proc Natl Acad Sci U S A, vol. 120, no. 6, pp. e2114204120, 2023, ISSN: 1091-6490.
