Featured Paper of the Month – December 2024
Published in Nature Communications Biology by András H Lékó, Adriana Gregory-Flores and Lorenzo Leggio et al. of the NIDA IRP Clinical Psychoneuroendocrinology and Neuropsychopharmacology Section.
Summary
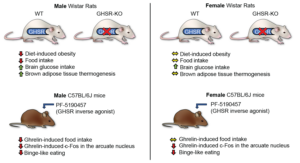
The stomach-derived ‘hunger hormone’ ghrelin plays an important role in metabolism, reward, and food intake. Serum ghrelin levels fluctuate along with feeding states, increasing during fasting and decreasing during satiety. The orexigenic and obesity-promoting effects of ghrelin are mediated by the growth hormone secretagogue receptor (GHSR; the ghrelin receptor), which is localized widely in the body, including the central nervous system and adipose tissue. High-fat diets can induce weight gain and obesity in rodents and humans. Here, we investigated whether the deletion of the GHSR gene (GHSR-KO) in rats can prevent or attenuate weight gain caused by a high-fat diet. Our main findings were that the GHSR gene deletion protected against obesity and decreased food intake in male but not female rats. GHSR gene deletion increased thermogenesis and brain glucose uptake and metabolism in a sex-specific manner. Using a pharmacological approach, we found that the inverse agonist/competitive antagonist PF-5190457 attenuated ghrelin-induced food intake in male but not in female mice. Our results support GHSR as a promising target for new pharmacotherapies for obesity; the sexual dimorphism highlighted in this study needs to be considered during medication development.
Image copyright: Nature Communications Biology
Publication Information
Genetic or pharmacological GHSR blockade has sexually dimorphic effects in rodents on a high-fat diet Journal Article
In: Commun Biol, vol. 7, no. 1, pp. 632, 2024, ISSN: 2399-3642.