Twice-Yearly Lenacapavir or Daily F/TAF for HIV Prevention in Cisgender Women
Published in The New England Journal of Medicine.
Authors
Linda-Gail Bekker, Moupali Das, Quarraisha Abdool Karim, Khatija Ahmed, Joanne Batting, William Brumskine, Katherine Gill, Ishana Harkoo, Manjeetha Jaggernath, Godfrey Kigozi, Noah Kiwanuka, Philip Kotze, Limakatso Lebina, Cheryl E Louw, Moelo Malahleha, Mmatsie Manentsa, Leila E Mansoor, Dhayendre Moodley, Vimla Naicker, Logashvari Naidoo, Megeshinee Naidoo, Gonasagrie Nair, Nkosiphile Ndlovu, Thesla Palanee-Phillips, Ravindre Panchia, Saresha Pillay, Disebo Potloane, Pearl Selepe, Nishanta Singh, Yashna Singh, Elizabeth Spooner, Amy M Ward, Zwelethu Zwane, Ramin Ebrahimi, Yang Zhao, Alexander Kintu, Chris Deaton, Christoph C Carter, Jared M Baeten, Flavia Matovu Kiweewa; PURPOSE 1 Study Team
Paper presented by Elliot Glotfelty, Ph.D. and selected by the NIDA TDI Paper of the Month Committee
Publication Brief Description
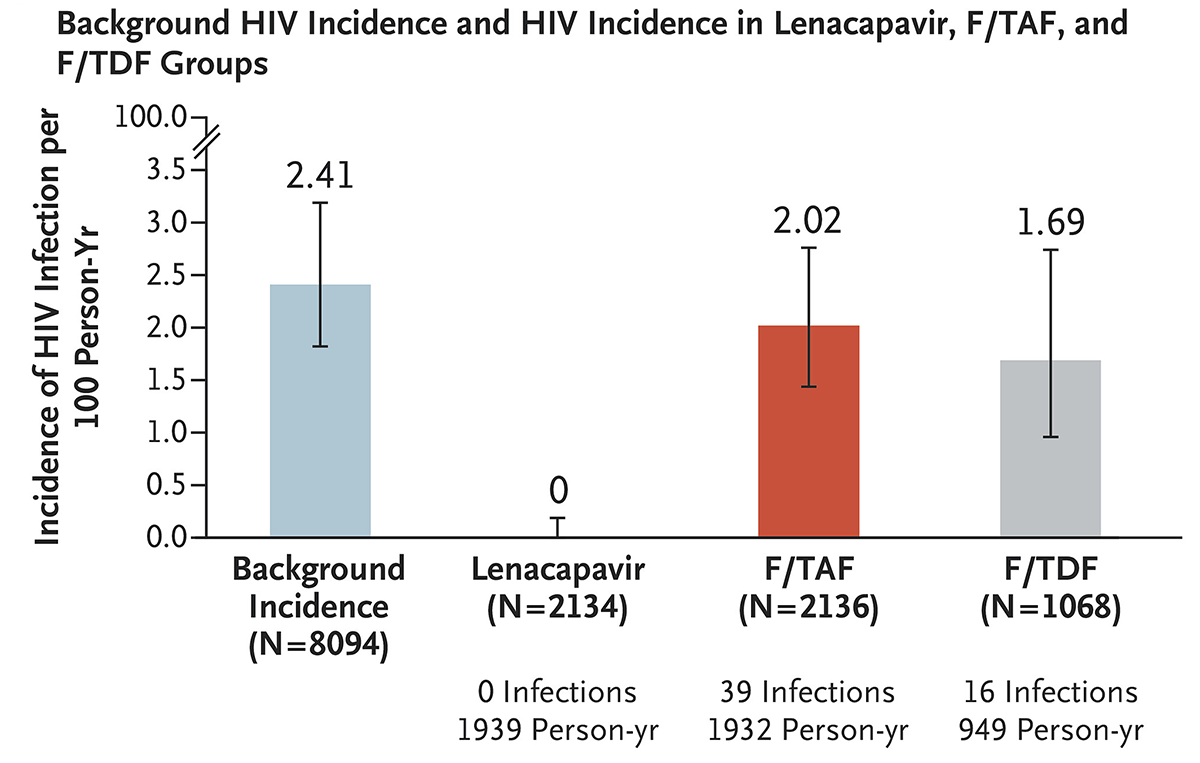
Patient adherence to a daily medication regimen is consistently one of the barriers to effective disease prevention and treatment. Current Food and Drug Administration (FDA) treatments/prophylactics for human immunodeficiency virus (HIV) require daily oral administration of medication to effectively prevent or quell HIV infection. A high risk population for HIV transmission includes those who inject illicit drugs, among other high risk behaviors, representing a subpopulation that could benefit from HIV harm reduction strategies that do not require daily oral medication. Bekker et al. (2024) presents a two-year phase 3, double blind, randomized, control trial involving adolescent girls and young women in South Africa and Uganda receiving either subcutaneous lenacapavir every 26 weeks, daily oral emtricitabine-tenofovir alafenamide (F/TAF), or daily oral emtricitabine-tenofovir disoproxil fumarate (F/TDF; active control). F/TAF and F/TDF are FDA approved preexposure prophylaxis (PrEP) antiretroviral drugs that require strict adherence to daily oral administration; lenacapavir is a novel, first in class, multistage HIV-1 capsid inhibitor with high potency and a long half-life allowing for twice yearly subcutaneous injection to maintain active drug bioavailability. All participants in this clinical trial were HIV-negative at the start of the study and were not receiving PrEP medications. Strikingly, no participants receiving twice-yearly lenacapavir acquired HIV infections, with HIV infections significantly lower than background HIV incidence and HIV incidence within the F/TDF group over the two year trial. Lenacapavir may be an effective harm reduction strategy for populations engaging in high risk behaviors prone to transmitting HIV, such as illicit injectable drug use, and those less likely to adhere to daily oral prophylactics.
Twice-Yearly Lenacapavir or Daily F/TAF for HIV Prevention in Cisgender Women Journal Article
In: N Engl J Med, vol. 391, no. 13, pp. 1179–1192, 2024, ISSN: 1533-4406.