Hot Off the Press! – March 2016
Lagerlöf, Olof; Slocomb, Julia E; Hong, Ingie; Aponte, Yeka; Blackshaw, Seth; Hart, Gerald W; Huganir, Richard L
The nutrient sensor OGT in PVN neurons regulates feeding. Journal Article
In: Science, vol. 351, no. 6279, pp. 1293–1296, 2016, ISSN: 1095-9203 (Electronic); 0036-8075 (Linking).
@article{Lagerlöf2016,
title = {The nutrient sensor OGT in PVN neurons regulates feeding.},
author = {Olof Lagerlöf and Julia E Slocomb and Ingie Hong and Yeka Aponte and Seth Blackshaw and Gerald W Hart and Richard L Huganir},
url = {https://www.ncbi.nlm.nih.gov/pubmed/26989246},
doi = {10.1126/science.aad5494},
issn = {1095-9203 (Electronic); 0036-8075 (Linking)},
year = {2016},
date = {2016-03-18},
journal = {Science},
volume = {351},
number = {6279},
pages = {1293--1296},
address = {Solomon H. Snyder Department of Neuroscience, Kavli Neuroscience Discovery Institute, Johns Hopkins University School of Medicine, Baltimore, MD 21205, USA. Department of Biological Chemistry, Johns Hopkins University School of Medicine, Baltimore, MD 21205, USA.},
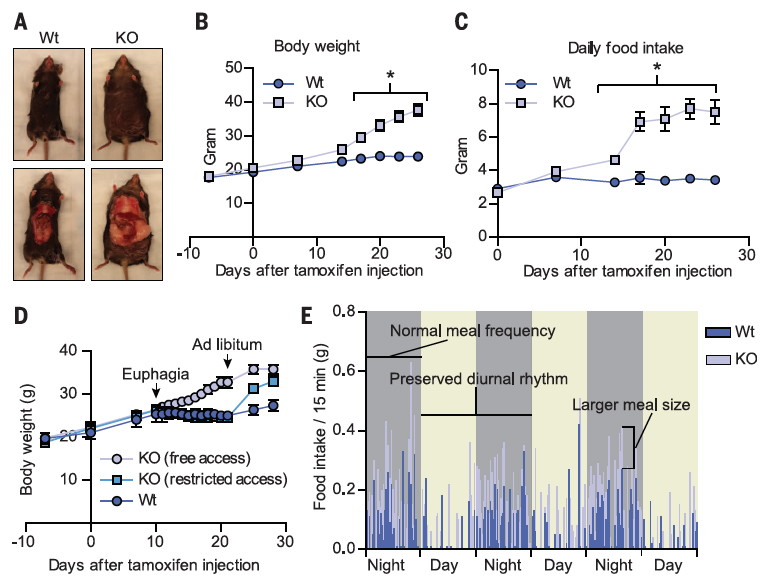
abstract = {Maintaining energy homeostasis is crucial for the survival and health of organisms. The brain regulates feeding by responding to dietary factors and metabolic signals from peripheral organs. It is unclear how the brain interprets these signals. O-GlcNAc transferase (OGT) catalyzes the posttranslational modification of proteins by O-GlcNAc and is regulated by nutrient access. Here, we show that acute deletion of OGT from alphaCaMKII-positive neurons in adult mice caused obesity from overeating. The hyperphagia derived from the paraventricular nucleus (PVN) of the hypothalamus, where loss of OGT was associated with impaired satiety. These results identify O-GlcNAcylation in alphaCaMKII neurons of the PVN as an important molecular mechanism that regulates feeding behavior.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Maintaining energy homeostasis is crucial for the survival and health of organisms. The brain regulates feeding by responding to dietary factors and metabolic signals from peripheral organs. It is unclear how the brain interprets these signals. O-GlcNAc transferase (OGT) catalyzes the posttranslational modification of proteins by O-GlcNAc and is regulated by nutrient access. Here, we show that acute deletion of OGT from alphaCaMKII-positive neurons in adult mice caused obesity from overeating. The hyperphagia derived from the paraventricular nucleus (PVN) of the hypothalamus, where loss of OGT was associated with impaired satiety. These results identify O-GlcNAcylation in alphaCaMKII neurons of the PVN as an important molecular mechanism that regulates feeding behavior.