 Featured Paper of the Month – November 2022
Featured Paper of the Month – November 2022
Published in Neuropsychopharmacology by Thomas Ross and Elliot Stein of the NIDA IRP Neuroimaging Research Branch.
Summary
The striatum, part of the forebrain, is critically involved in reward processes and substance use. Many of the inputs to the striatum come from frontal brain regions. One way to study these connections in living humans is using a technique called functional magnetic resonance imaging (fMRI). A common fMRI approach, resting-state functional connectivity, typically examines the temporal similarity of the signals from two brain areas: essentially looking at the connection between ‘point A’ and ‘point B.’ However, we know from animal studies and from anatomical tissue studies that the striatum receives inputs from many frontal regions simultaneously. Thus, the typical A-to-B analysis approach is likely a poor representation of what is happening in the brain.
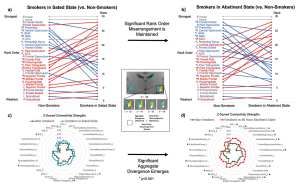
In this paper, NIDA scientists created and implemented a novel analysis approach that they termed “connectivity profile analysis,” or CPA. This approach looks at the connections from all frontal regions to each striatal region. Using CPA, they are able to look for 3 types of misconfigurations: an overall difference in the strengths of connections, a change in the order of connection strengths and a change in the diversity of the connections.
This CPA approach was applied to people with nicotine use disorder (NUD) under two conditions, while on nicotine and after about 2 days abstinent, compared to a non-smoking group. They found two different misconfigurations in people with NUD. In the top (dorsal) region of the striatum, the order of connection strengths differed between people with NUD and the comparison group. This difference was present whether people with NUD were on nicotine or were abstinent. However, in the bottom (ventral) portion of the striatum there was a difference in overall strength of connections, but only when examining the people with NUD in the abstinent condition.
These findings underscore the potential for approaches that better model the biology of brain connections to yield deeper insights into the neural basis of substance use disorders. For example, the identified sites of misconfiguration could serve as useful targets for therapies like transcranial magnetic stimulation and/or as biomarker readouts for treatment efficacy.
Publication Information
Misconfigured striatal connectivity profiles in smokers Journal Article
In: Neuropsychopharmacology, vol. 47, no. 12, pp. 2081–2089, 2022, ISSN: 1740-634X.
