 Chemogenetics-based neurotheranostics
Chemogenetics-based neurotheranostics
We are developing and characterizing novel, selective, and potent neurotheranostic ligands for chemogenetic technologies and combining their use with translational molecular imaging.
Figure (right): Precision medicine offers significant advantages over conventional medical treatment. Within precision medicine, theranostics comprises a strategy that combines THERApeutic and diagNOSTIC strategies to provide a personalized treatment approach encompassing disease diagnosis, drug delivery, and disease/therapy monitoring using a single agent. Theranostic strategies confer improvement in treatment efficacy compared to conventional medicine and afford significant promise for precision psychiatry and neurology. Such neurotheranostic interventions are particularly timely given recent developments in neuromodulatory technologies. One such technology, called chemogenetics, offers the unprecedented ability to control neuronal activity in a cell type-specific manner in the freely-moving subject, without the need for chronically-implantable devices. A key feature of chemogenetic technologies is that they can be combined with clinical molecular imaging diagnostic methods such as positron emission tomography (PET). This particular combination extends the therapeutic component of chemogenetics to encompass its use in precision medicine-based neurotheranostics.
Related Publications
- Gomez, Bonaventura et al. 2017
- Magnus, Lee et al., 2019
- Bonaventura, Eldridge, Hu, et al., 2019
- Fleury Curado, et al., 2020
- Hu, Morris Bonaventura, et al., 2020
- Boehm, et al., 2021
 Optogenetics-based neurotheranostics
Optogenetics-based neurotheranostics
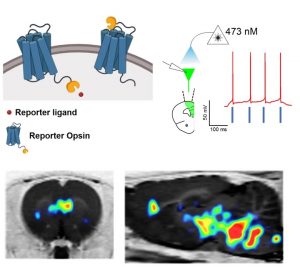
Optogenetics is a revolutionary technology that consists of unique ion channels expressed in neural tissue which upon light stimulation can either activate or inhibit neurons with exquisite temporal precision. Optogenetics has had a tremendous impact on basic neuroscience research. However, its impact in translational brain applications has been limited. One reason for this, is that to date, opsins have not been able to be visualized noninvasively in intact subjects. As such we are working on a noninvasive reporter detection system for optogenetics. This scalable system consists of chimeric opsins tagged with a small protein epitope and a clinically-used PET radioligand and permits noninvasive, quantitative, and longitudinal detection of opsins in the brain in translational and potentially, also in clinical applications.
Figure (right): A novel reporter opsin called ChRERa elicits light-driven neuronal activation and can be visualized noninvasively in cells bodies and at their terminal projections sites using PET.
For more details our “Hot Topics” presentation at the ACNP 2019 meeting can be found here.
 Noninvasive, quantitative, and longitudinal cell type-specific mapping of brain activity
Noninvasive, quantitative, and longitudinal cell type-specific mapping of brain activity
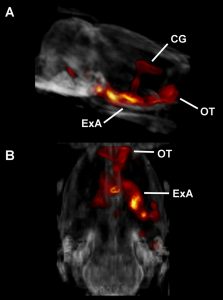
We implement chemogenetics, optogenetics, pharmacological, magnetic, or electrical stimulation with PET imaging in awake, freely-moving animals either in an exploratory fashion, to determine whole-brain functional networks recruited during behaviorally-relevant contexts, or to corroborate functional connectivity of a defined neuron-type, region, pathway or peripheral tissue/organ. We use such approaches to map functional anatomy related to a variety of cell-types/projections in distinct brain regions in basic and translational research.
Figure (right): DREADD-assisted metabolic mapping (DREAMM) showing metabolic activation of a brain network comprising cingulate gyrus (CG), olfactory tubercle (OT) and extended amygdala (ExA) in response to DREADD-mediated inhibition of prodynorphin-expressing neurons in a discrete subregion of the amygdala, the periamygdaloid cortex.
Related Publications
- Michaelides et al., 2013
- Anderson et al., 2013
- Michaelides & Hurd, 2014
- Urban et al., 20
- Mazzone et al., 2016
- Bonaventura, Eldridge, Hu, et al., 2019
- Fleury Curado et al., 2020
![PET image coregistered to MRI showing non-invasive assessment of dopamine D2/D3 receptors using [11C]raclopride in mouse striatum.](https://irp.nida.nih.gov/wp-content/uploads/2021/03/biobehavioral_300.gif) Biobehavioral molecular imaging
Biobehavioral molecular imaging
A considerable portion of our research program relies on the use of advanced quantitative molecular imaging via positron emission tomography (PET). We perform PET studies by employing a variety of radioligands depending on experimental need. These can be procured commercially or are custom made to perform noninvasive and longitudinal assessments of brain metabolic activity, neuroinflammation, neurotransmitter displacement, and receptor occupancy/target engagement of candidate compounds or other processes.
Figure (right): PET image coregistered to MRI showing non-invasive assessment of dopamine D2/D3 receptors using [11C]raclopride in mouse striatum.
Related Publications
 Development of molecular imaging PET probes
Development of molecular imaging PET probes
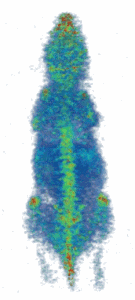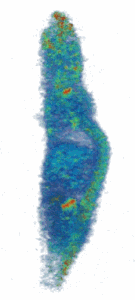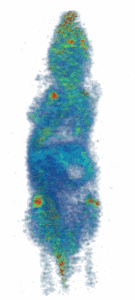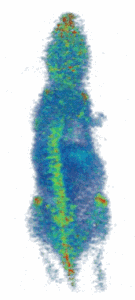
We are developing novel imaging agents & applications for endogenous targets including non-invasive visualization of mu opioid receptors and elemental Zn2+
Figure (right): Noninvasive imaging of elemental trace zinc uptake throughout the body of a mouse. High levels of zinc are observed in the brain, spine, nasal area, joints, and liver.
Related Publications
 Trace metals and addiction – Zinc potentiates dopamine neurotransmission and cocaine seeking
Trace metals and addiction – Zinc potentiates dopamine neurotransmission and cocaine seeking
Metals such as Fe, Zn and Cu are essential for normal neurobiological functioning and many disease states are characterized by metal imbalances. However, the precise involvement of such metals in normal neurobiology, and in disease states like addiction are not well understood. Zn specifically is transported into synaptic vesicles via its ZnT3 transporter and is co-released from presynaptic terminals along with glutamate but its function is not well understood. We are currently exploring a role for Zn and ZnT3-positive neurons and circuits on in vivo dopamine neurotransmission, cocaine abuse vulnerability, and motivated behaviors.
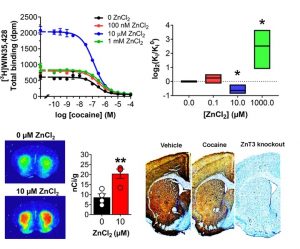
Figure (right): At physiological concentrations, Zn increases the affinity of cocaine at its main endogenous target, the dopamine transporter. Cocaine also decreases synaptic Zn2+ levels in the brain.
Related Publications
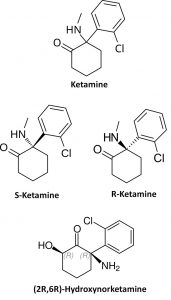 Characterization of drugs of abuse – focus on ketamine enantiomers & metabolites
Characterization of drugs of abuse – focus on ketamine enantiomers & metabolites
Ketamine is a controlled substance, has abuse potential, and can induce undesirable side effects. Nevertheless, it is considered to be generally safe and is a widely-used dissociative anesthetic and rapid-acting pain medication. The recent discovery that a single subanesthetic dose of ketamine (a racemic mixture in equal proportion of S-ketamine and R-ketamine enantiomers) produces rapid and long-lasting antidepressant effects in individuals with major depressive disorder (MDD), bipolar depression, and treatment-resistant depression (TRD) has been hailed as a key psychiatric breakthrough. Ketamine is generally regarded as a non-competitive N-methyl-D-aspartate receptor (NMDAR) antagonist but also shows considerable affinity for other receptors. Though preclinical studies have investigated ketamine pharmacology and abuse liability, no study to date has characterized the precise in vitro and in vivo pharmacological properties and the abuse liability of its enantiomers. S-ketamine (esketamine, SpravatoTM) was recently approved by the FDA as an intranasal formulation for TRD and human trials assessing efficacy of R-ketamine in depression are currently underway. As depression shares strong comorbidity with substance use disorders, we are working to better understand the abuse liability of each enantiomer.
A ketamine metabolite, (2R,6R)-hydroxynorketamine (HNK), has been recently implicated in underlying ketamine’s efficacy in preclinical models of depression. We are also working on the pharmacological characterization of HNK, for which clinical trials are also underway.
Figure (right): Structures of racemic ketamine, its enantiomers, and its (2R, 6R)-HNK metabolite
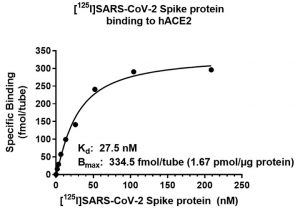 SARS-CoV-2 and COVID-19
SARS-CoV-2 and COVID-19
Our labs specializes in discovery and characterization of drug-receptor and protein-receptor interactions. As such we are using our capabilities to identify novel receptors for the SARS-CoV-2 Spike protein.
Figure (Right): Saturation binding assay assessing radiolabeled I125-Spike against recombinant human ACE2.
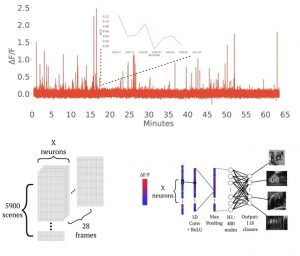 Neuroscience & artificial intelligence
Neuroscience & artificial intelligence
We are exploring the interface of neuroscience and AI by leveraging the Brain Observatory dataset from the Allen Institute to examine the performance of novel machine and deep learning neural network architectures at decoding visual stimuli from calcium data derived from the parcellated visual cortex of the mouse. This approach is also yielding interesting novel facets of the biological properties of visual stimulus encoding by discrete cell types of the visual cortex. Our recent preprint describing the use of deep learning for visual decoding can be found here.
Figure (Right): Example GCaMP6f trace data alongside a representative machine learning architecture we are implementing for visual stimulus decoding using neuronal calcium data derived from discrete cell-types.
